Use of notoginsenoside Ft1
A technology of notoginseng saponins and uses, applied in the field of medicine, can solve problems such as recurrent attacks, achieve the effect of improving weight loss and good application prospects
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0042] 1. Experimental materials
[0043] 1.1 Medicinal materials
[0044] Notoginseng saponin Ft1 (HPLC purity ≥98%, Chengdu Master Biotechnology Co., Ltd.); Dextran sodium sulfate (DSS, CAS: 9011-18-1, MW36-50kDa, produced by MP Biomedicals, USA); Willow Azasulfapyridine (SASP, CAS: 599-79-1, molecular weight 398.39, HPLC purity ≥ 98%, produced by Sigma-Aldrich, USA).
[0045] 1.2 Experimental animals
[0046] C57BL / 6 female mice (8 weeks) weighing 20±2 g were provided by the Experimental Animal Center of Shanghai University of Traditional Chinese Medicine, animal qualification certificate number: SYXK (Shanghai) 2009-0069. Placed in a conventional feeding environment, free access to food and water.
[0047] 2. Experimental method
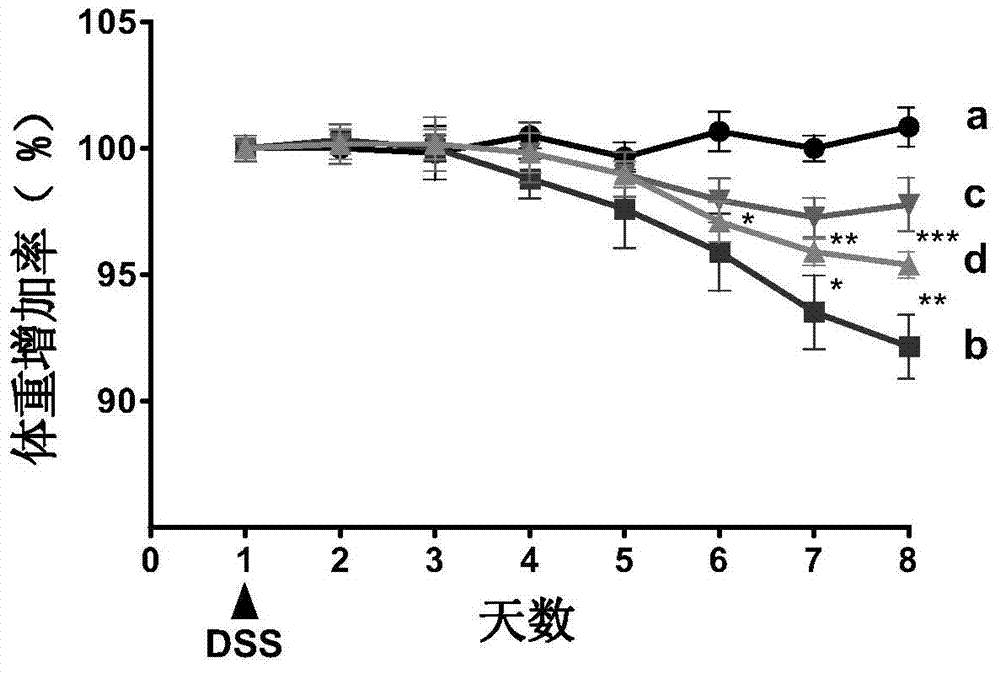
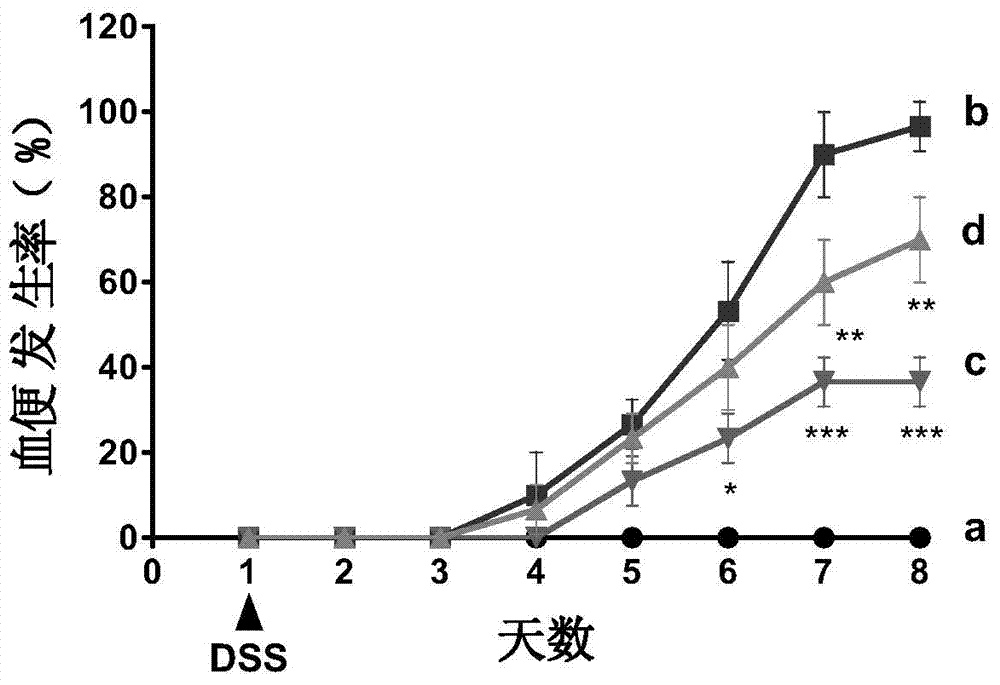
[0048] 2.1 Establishment of ulcerative colitis model
[0049] C57BL / 6 female mice (20±2g) with uniform body weight were selected, and the internationally accepted method for modeling ulcerative colitis (Gastroenterology2002, 123:256-70; PLoS...
Embodiment 2
[0073] The preparation of embodiment 2 tablet
[0074] Utilize conventional technique, mix following component, then directly compress tablet, prepare the pharmaceutical composition of tablet form, its formula is as follows:
[0075]
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com