Macrocyclic Inhibitors of Flaviviridae Viruses
A compound, methyl technology, applied in the field of therapy
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
preparation example Construction
[0249] Preparation of macrocycles
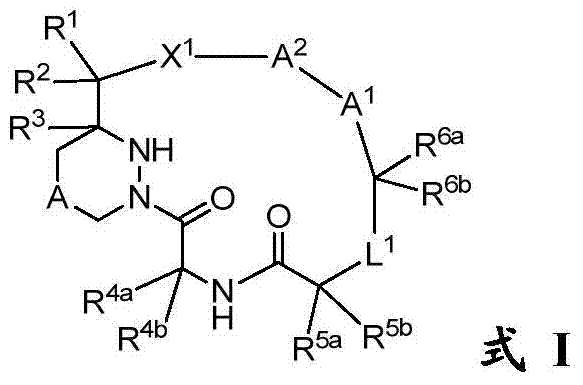
[0250] Compounds of the invention, such as those of general formula I, II, II-a, II-b, II-c or III, can be prepared according to the schemes described below, but it will be appreciated that the illustrated methods or other method improvement. As illustrated in Scheme 1, from the five key components A-E, the protective group (PG 1 -PG 8 ), and combine them in order to synthesize the macrocyclic compound M. The dashed lines numbered 1-5, referred to herein as connection 1, connection 2, etc. respectively, are the 5 connections used to bind components A-E. The order in which a particular linkage occurs can vary and depends on the choice of protecting group and the desired chemistry. Typically, ligation 3, 4 or 5 is used as the final macrocyclization step.
[0251] plan 1
[0252]
[0253] Links 1 and 2 are amide bonds. Linkages are formed between the respective acids and amines using standard peptide coupling reagents known to those s...
Embodiment 1
[0467] Example 1: (E)-(2R,3R)-7-(3-{(R)-1-[((S)-hexahydro-pyridazine-3-carbonyl)-amino]-ethyl}- Phenyl)-3-methoxy-2-methyl-hept-6-enoic acid (S)-2-methyl-1-((S)-1-methyl-2-oxo-ethylaminomethyl acyl)-propyl ester- compound 1
[0468]
[0469] Synthesis of compound 1a: Preparation of 1-((1R,5S)-10,10-dimethyl-3,3-dioxo-3λ*6*-thio-4-aza-tricyclo in toluene (50 mL) A solution of [5.2.1.0*1,5*]dec-4-yl)-propan-1-one (3.95 g, 14.55 mmol) was then evaporated to dryness. This process was repeated, and the resulting white solid was dissolved in anhydrous dichloromethane (16 mL). A small amount of calcium hydride was added, followed by tert-butyldimethylsilyl trifluoromethanesulfonate (3.83 mL, 14.5 mmol) and anhydrous triethylamine (2.33 mL, 16.7 mmol). The reaction mixture was stirred at room temperature ("RT") for 15 hours ("h") under a nitrogen atmosphere. The resulting solution was evaporated to yield a thick paste which was redissolved in anhydrous dichloromethane (15 mL) a...
Embodiment 2
[0492] Embodiment 2: compound 2
[0493]
[0494] Synthesis of compound 2a: Preparation of 1-((1R,5S)-10,10-dimethyl-3,3-dioxo-3λ*6*-thia-4-nitrogen in anhydrous dichloromethane (24ml) A solution of hetero-tricyclo[5.2.1.0*1,5*]dec-4-yl)-propan-1-one (6.0 g, 22.1 mmol) and addition of tert-butyldimethylsilyltrifluoromethanesulfonic acid (5.0 mL, 22.1 mmol), followed by the addition of anhydrous triethylamine (3.54 mL, 25.4 mmol). The reaction mixture was stirred at room temperature under nitrogen atmosphere for 15 h. This gave a dark solution which evaporated to an oil. The oil was dissolved in anhydrous dichloromethane (22 mL), and the solution was added dropwise to crotonaldehyde (3.66 mL, 44.2 mmol) and Titanium tetrachloride (1M in dichloromethane, 44.2 mL, 44.2 mmol). The reaction mixture was stirred at -78 °C for 1 h, then ammonium chloride solution (30 mL) was added. The reaction mixture was allowed to warm to room temperature and the layers were separated. The...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com