Cyclodextrin-base comb polypseudorotaxane (CD-PPR) aquagel and preparation method thereof
A technology of polypseudorotaxanes and hydrogels, which is applied in the field of cyclodextrin-based comb polypseudorotaxane hydrogels, and can solve the problems of not using side chain cyclodextrin-based polypseudorotaxanes
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
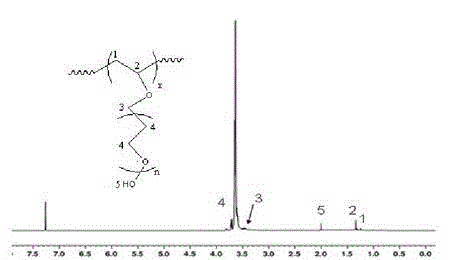
[0021] Step a: Weigh 15 g of APEG-1200 and dissolve it in 20 mL of redistilled DMF solvent, then add 0.3 g of AIBN, react under nitrogen protection at 65°C for 15 h, and distill off the solvent under reduced pressure at 80°C after the reaction , and finally dried under vacuum at 60°C to obtain APEG-1200 polymer. Weigh 5 g of APEG-1200 polymer and dissolve it in 50 mL of water, extract with 150 mL of chloroform, and repeat the operation three times.
[0022] Step b: Dissolve 0.1 g of the extracted polymer in 1 mL of water, add dropwise to the aqueous solution of 0.03 g / ml α-CD, sonicate for 15 min to make it evenly mixed, and then let the system stand for 12 h, A hydrogel is formed. At this time, the polyoxyethylene segment (CH 2 CH 2 O) to the molar ratio of α-CD (n(CH 2 CH 2 O) / n(α-CD)) is 6.
Embodiment 2
[0024] Step a: Weigh 15 g of APEG-1200 and dissolve it in 20 mL of redistilled DMF solvent, then add 0.6 g of AIBN, react under nitrogen protection at 70°C for 15 h, and distill off the solvent under reduced pressure at 80°C after the reaction , and finally dried under vacuum at 60°C to obtain APEG-1200 polymer. Weigh 5 g of APEG-1200 polymer and dissolve it in 50 mL of water, extract with 150 mL of chloroform, and repeat the operation three times.
[0025] Step b: Dissolve 0.1 g of the extracted polymer in 1 mL of water, add dropwise to the α-CD aqueous solution with a concentration of 0.05 g / ml, sonicate for 15 min to make it evenly mixed, and then let the system stand for 12 h. A hydrogel is formed. At this time, the polyoxyethylene segment (CH 2 CH 2 O) to the molar ratio of α-CD (n(CH 2 CH 2 O) / n(α-CD)) is 4.
Embodiment 3
[0027] Step a: Weigh 15 g APEG-1200 and dissolve it in 20 mL redistilled DMF solvent, then add 0.9 g AIBN, react under nitrogen protection at 75 °C for 15 h, and distill off the solvent under reduced pressure at 80 °C after the reaction , and finally dried under vacuum at 60°C to obtain APEG-1200 polymer. Weigh 5 g of APEG-1200 polymer and dissolve it in 50 mL of water, extract with 150 mL of chloroform, and repeat the operation three times.
[0028] Step b: Dissolve 0.1 g of the extracted polymer in 1 mL of water, add dropwise to the α-CD aqueous solution with a concentration of 0.09 g / ml, sonicate for 15 min to make it evenly mixed, and then let the system stand for 12 h. A hydrogel is formed. At this time, the polyoxyethylene segment (CH 2 CH 2 O) to the molar ratio of α-CD (n(CH 2 CH 2 O) / n(α-CD)) is 2.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com