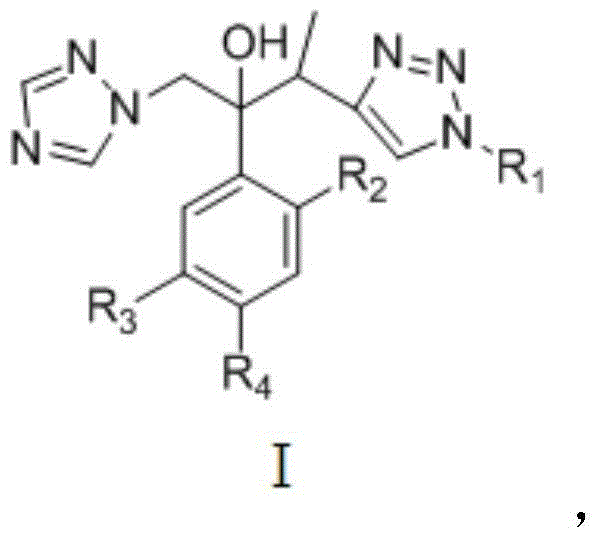
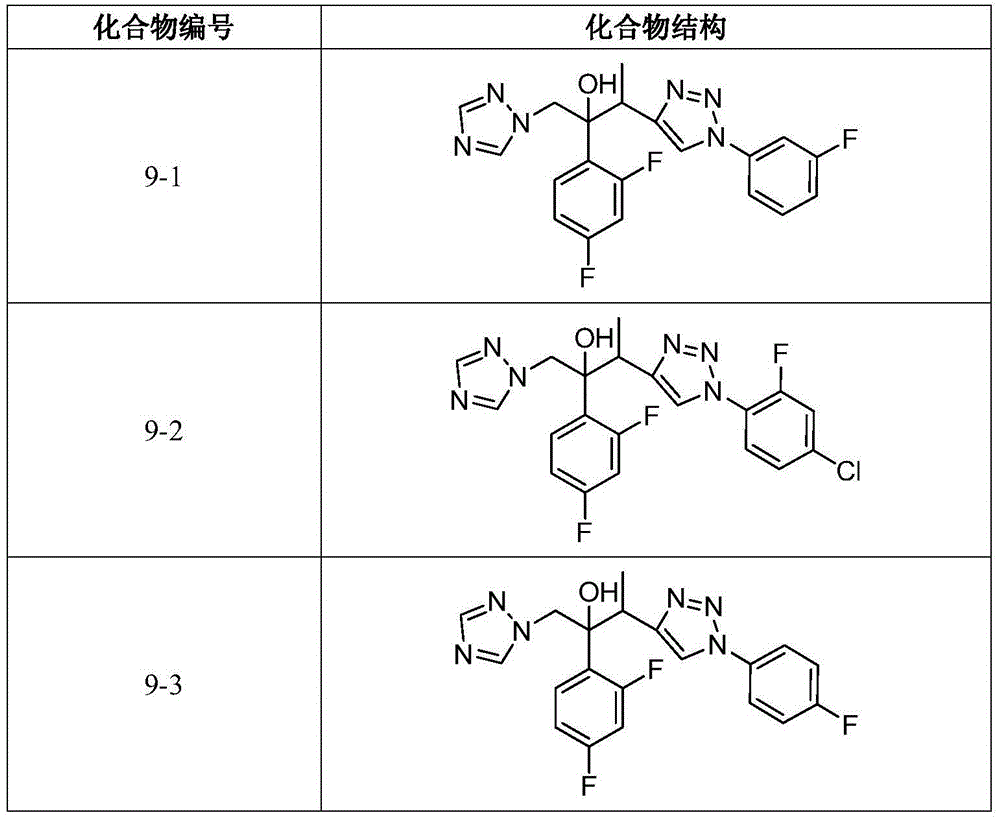
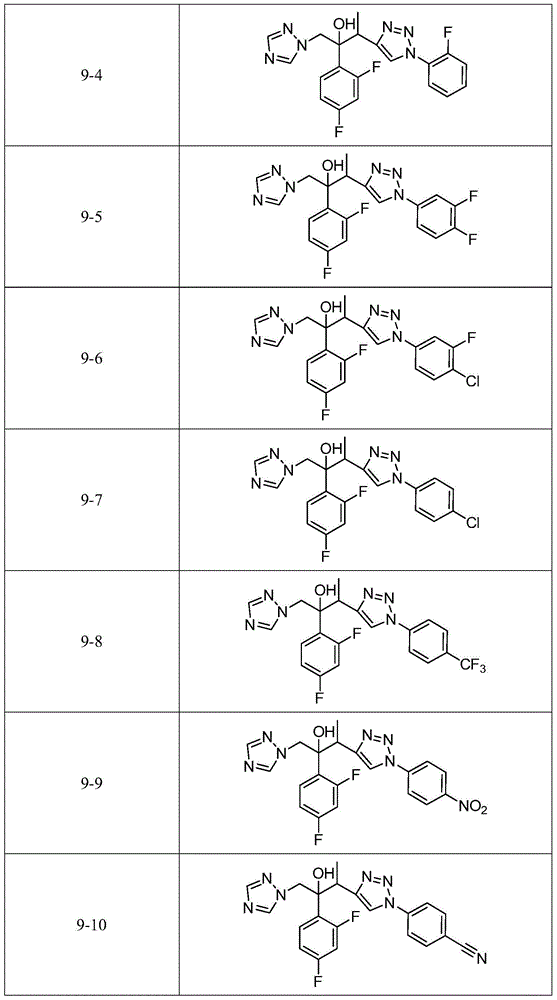
Triazole alcohol derivative, preparation method and application thereof
A kind of technology of triazole alcohol and triazole, applied in the field of medicinal compounds
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0082] Example 1: (2R*,3S*)-2-(2,4-difluorophenyl)-3-methyl-1-(1H-1,2,4-triazol-1-yl)- Synthesis of 4-pentyn-2-ol
[0083] Step 1: Synthesis of 3-butyn-2-ol mesylate
[0084] Dissolve 3-butyn-2-ol (25g) in dichloromethane, add triethylamine (99mL), add methanesulfonyl chloride (41mL) dropwise at -78 degrees, after the reaction is complete, pour 200mL of bicarbonate In sodium aqueous solution, extracted twice with dichloromethane, the organic phase was washed twice with water, dried with anhydrous sodium sulfate, spin-dried and used directly in the next step.
[0085] NMR data: 1 H NMR (300MHz, CDCl 3 )δ5.29(q,1H),3.13(s,3H),2.71(s,1H),1.66(d,3H).
[0086] Step 2: Synthesis of (2R*,3S*)-1-chloro-2-(2,4-difluorophenyl)-3-methyl-4-pentyn-2-ol
[0087] Pd(CH 3 EN) 2 Cl 2 Dissolve in tetrahydrofuran, add triphenylphosphine, add 2-chloro-2',4'-difluoroacetophenone and 3-butyn-2-ol methanesulfonate after reacting for 10 minutes, then slowly add di The toluene solution of eth...
Embodiment 2
[0091] Example 2: (2R*,3S*)-2-(2,4-dichlorophenyl)-3-methyl-1-(1H-1,2,4-triazol-1-yl)- Synthesis of 4-pentyn-2-ol
[0092] Step 1: Synthesis of (2R*,3S*)-1-chloro-2-(2,4-dichlorophenyl)-3-methyl-4-pentyne 2-ol
[0093] Pd(CH 3 EN) 2 Cl 2 Dissolve in tetrahydrofuran, add triphenylphosphine, add 2-chloro-2',4'-dichloroacetophenone and 3-butyn-2-ol mesylate after reacting for 10 minutes, then slowly drop di The toluene solution of ethyl zinc was poured into the hydrochloric acid solution after reacting at room temperature for 1 hour, extracted 3 times with ethyl acetate, the organic phase was washed twice with saturated aqueous sodium chloride solution, dried by anhydrous sodium sulfate and then spin-dried to obtain ( 2R*,3S*)-1-Chloro-2-(2,4-dichlorophenyl)-3-methyl-4-pentyn 2-ol crude. used directly in the next step.
[0094] Step 2: (2R*,3S*)-2-(2,4-dichlorophenyl)-3-methyl-1-(1H-1,2,4-triazol-1-yl)-4 -Synthesis of pentyn-2-ol
[0095] Dissolve 1H-1,2,4-triazole in DMS...
Embodiment 3
[0097] Example 3: (2R*,3S*)-2-(2,5-difluorophenyl)-3-methyl-1-(1H-1,2,4-triazol-1-yl)- Synthesis of 4-pentyn-2-ol
[0098] Step 1: Synthesis of (2R*,3S*)-1-chloro-2-(2,5-difluorophenyl)-3-methyl-4-pentyne 2-ol
[0099] Pd(CH 3 EN) 2 Cl 2 Dissolve in tetrahydrofuran, add triphenylphosphine, add 2-chloro-2', 5'-difluoroacetophenone and 3-butyn-2-ol mesylate after reacting for 10 minutes, then slowly add di The toluene solution of ethyl zinc was poured into the hydrochloric acid solution after reacting at room temperature for 1 hour, extracted 3 times with ethyl acetate, the organic phase was washed twice with saturated aqueous sodium chloride solution, dried by anhydrous sodium sulfate and then spin-dried to obtain ( 2R*,3S*)-1-Chloro-2-(2,5-difluorophenyl)-3-methyl-4-pentyn 2-ol crude. used directly in the next step.
[0100] Step 2: (2R*,3S*)-2-(2,5-difluorophenyl)-3-methyl-1-(1H-1,2,4-triazol-1-yl)-4 -Synthesis of pentyn-2-ol
[0101] Dissolve 1H-1,2,4-triazole in DMS...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com