Spiropyran photochromic compound and preparation method thereof
A compound and cyclization technology, applied in chemical instruments and methods, color-changing fluorescent materials, organic chemistry, etc., can solve problems such as high cost, unsuitable for large-area light-colored glass, and restrictions on commercial applications in the construction field
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
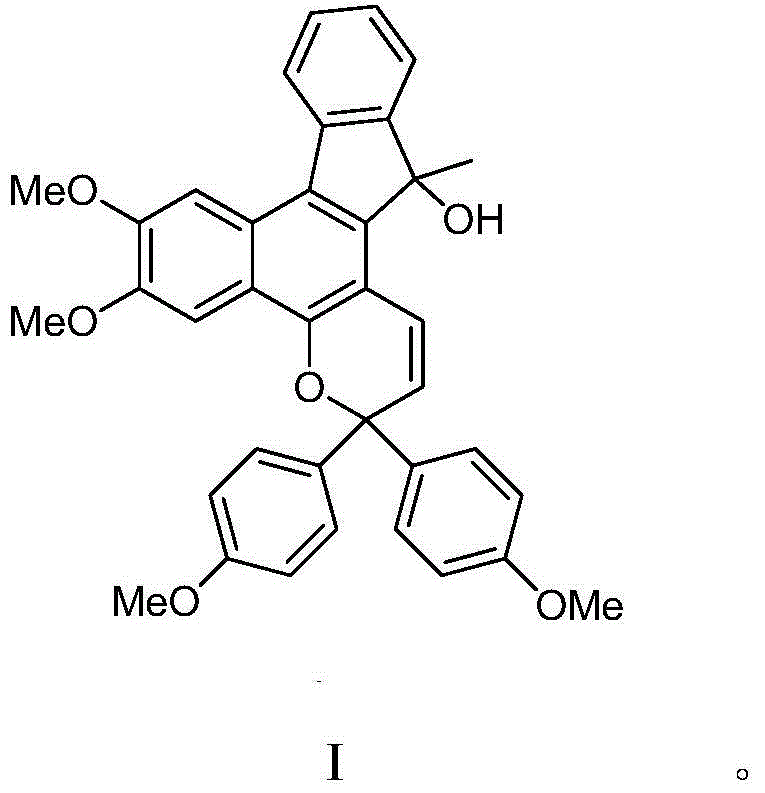
[0038] Example 1 Preparation of 6,7-dimethoxy-3,3-bis(4-methoxyphenyl)-13-methyl-3,13-dihydrobenzo[h]indene[2,1- f] pyran-13-ol
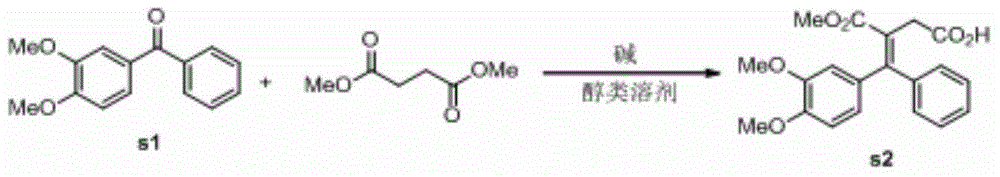
[0039] 1. The preparation of compound s2, the reaction formula:
[0040]
[0041] In a 500mL three-necked flask, add 3,4-dimethoxybenzophenone (50.0g, 206mmol, 1.0eq), potassium tert-butoxide (46.3g, 413mmol, 2.0eq), tert-butanol (150mL), drop Dimethyl succinate (36.2g, 248mmol, 1.2eq) was added, and after the addition was completed, the reaction temperature was raised to 80°C for 20h. Add 100 mL of water, distill off tert-butanol, then add 100 mL of water, adjust the pH of the aqueous layer to acidic, extract with dichloromethane (80 mL×3), combine the organic layers, and anhydrous Na2 SO 4 After drying, the crude product was directly used for the next reaction after the solvent was distilled off under reduced pressure.
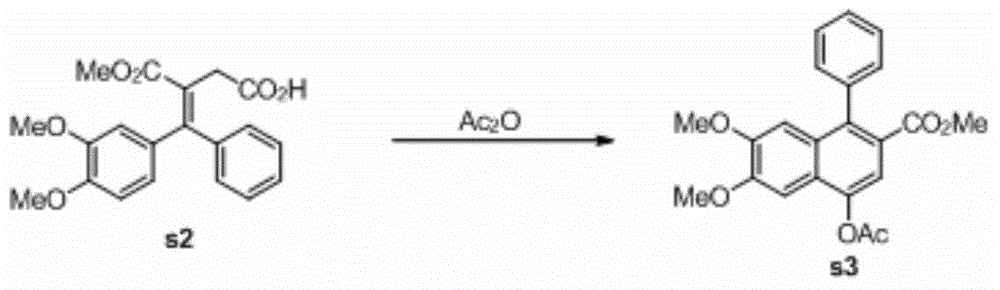
[0042] 2. The preparation of compound s3, the reaction formula:
[0043]
[0044] The crude product prepared in step 1...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com