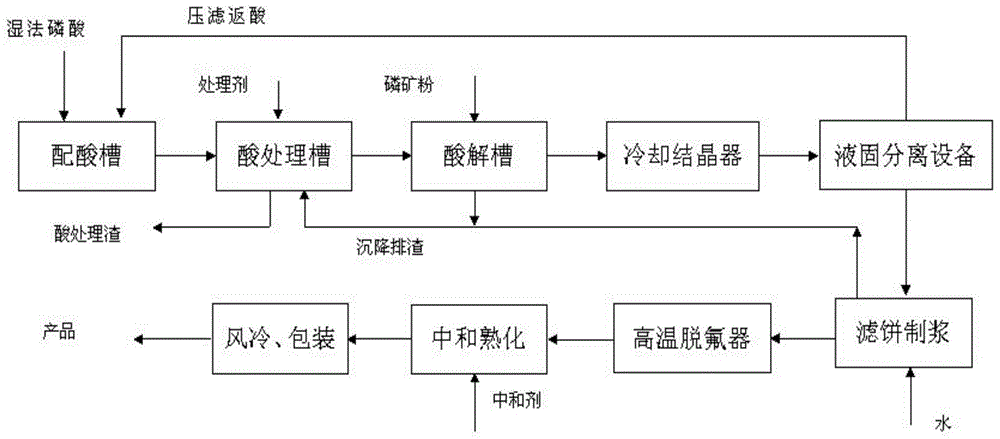
Process of producing feed-grade calcium dihydrogen phosphate through phosphoric acid circulation impurity removal method
A technology of calcium dihydrogen phosphate and feed grade, which is applied in the field of production of feed grade calcium dihydrogen phosphate by the phosphoric acid cycle impurity removal method, which can solve problems such as waste of resources, and achieve the effects of saving costs, improving water solubility, and reducing emissions
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0083] The phosphate rock in Kaiyang, Guizhou was used as the raw material for the experiment, and the raw material indicators are shown in Table 1:
[0084] Table 1 Raw material index of phosphate rock in Kaiyang area, Guizhou
[0085] indicators
[0086] The temperature of phosphoric acid for reaction is raised to 60-80°C in the circulating heater, and treatment agent 1 (Na 2 S or P 2 S 5 ) and treatment agent 2 (prepared in advance, the composition is similar to the sedimentation impurities described in the present invention), and a small amount of white carbon black is added as a filter aid at the same time. React for 30 minutes and filter; the filter residue is washed countercurrently with a small amount of clean water, and the P in the residue is detected. 2 o 5 The content is 1.1%. Add the filtrate acid into the neutralization reactor and raise the temperature to 80-100°C, start feeding the ore, and detect the H in the slurry 3 PO 4 The concentration co...
Embodiment 2
[0088] The raw ore of a phosphate mine in Kunyang, Yunnan Province was used as the raw material for the experiment, and the indicators are shown in Table 2:
[0089] Table 2 Raw material indicators of a phosphate mine in Kunyang, Yunnan
[0090] indicators
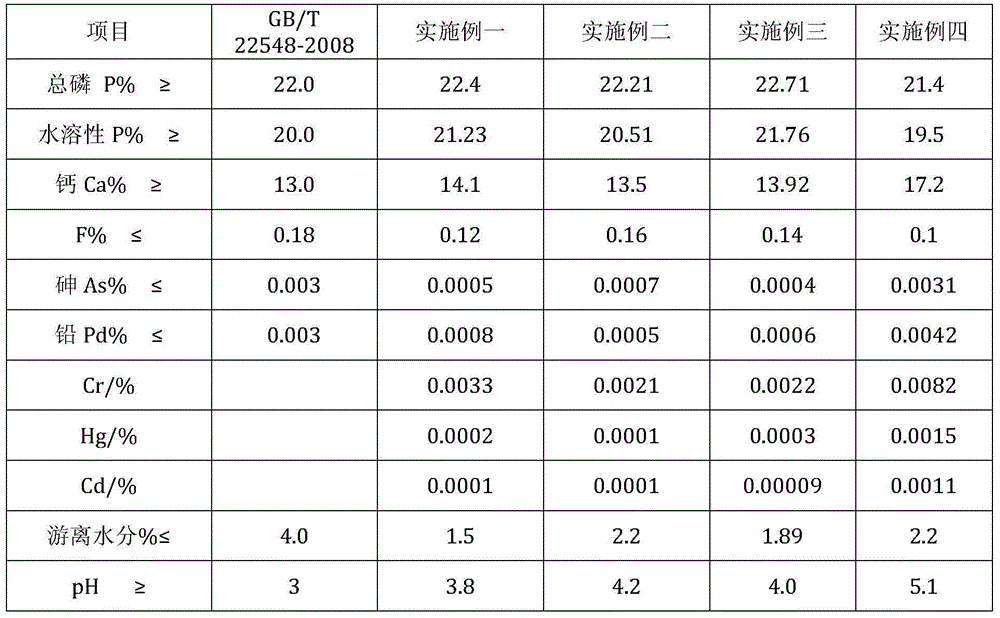
[0091] After the ore powder is ground, it participates in the reaction, and the process control is the same as in Example 1. The product indicators are shown in Table 4.
Embodiment 3
[0093]Taking a phosphate rock in Fuquan area, Guizhou, after flotation experiment, the indicators are shown in Table 3:
[0094] Table 3 Raw Material Indicators of Guizhou Fuquan Phosphate Concentrate
[0095] indicators
[0096] The process control is the same as that in Example 1, and the filter press acid is recycled for 72 hours of continuous operation, and the final product is comprehensively sampled, and the indicators are as shown in Table 4.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com