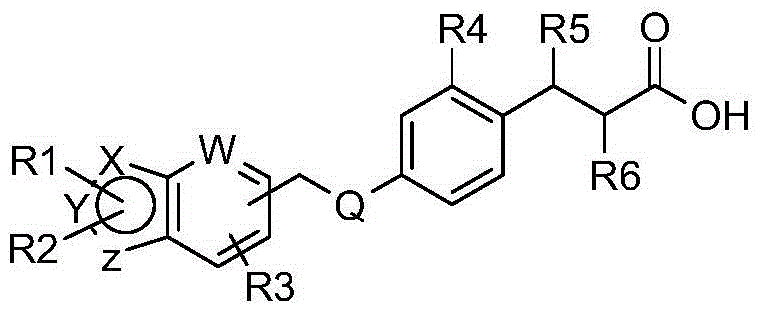
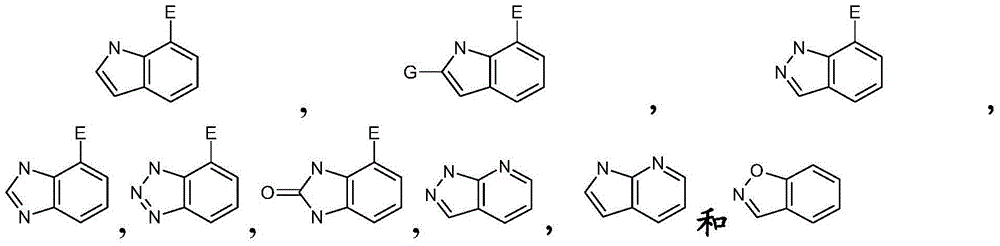
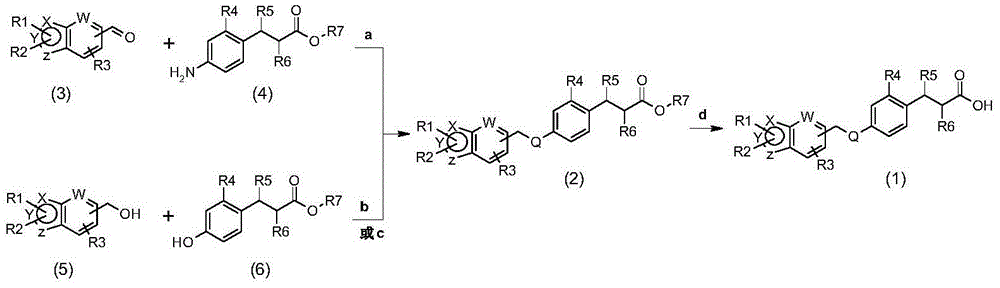
GPR40 receptor agonist, methods of preparing the same, and pharmaceutical compositions containing same as active ingredient
A compound and pharmaceutical technology, applied in the field of GPR40 receptor agonist, preparation thereof and pharmaceutical composition containing it as an active ingredient, can solve problems such as weight gain, weak hypoglycemia function, gastrointestinal disorders, etc.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
preparation Embodiment 1
[0464] Preparation Example 1: Synthesis of 1-isopropyl-1H-indole-6-carbaldehyde
[0465] 1-H-Indole-6-carbaldehyde (320 mg, 2.2 mmol) was dissolved in dimethylformamide (2 ml), to which was slowly added isopropyl iodide (0.33 ml, 3.3 mmol) and sodium hydride ( 104 mg, 2.6 mmol), then the mixture was stirred at 50 °C for 8 h. A 1N hydrochloric acid solution was added thereto, and the mixture was extracted with ethyl acetate. The extract was washed with saturated aqueous sodium chloride solution, dried over anhydrous magnesium sulfate, and filtered. The filtrate was distilled under reduced pressure and separated by column chromatography to obtain the title compound (300 mg, 73%).
[0466] NMR: 1 H-NMR (400 HMz, CDCl 3 ); δ10.11(s, 1H), 7.96(s, 1H), 7.71(d 1H), 7.62(dd, 1H), 7.45(d, 1H), 6.59(d, 1H), 4.73-4.84(m , 1H), 1.57(d, 6H)
preparation Embodiment 2
[0467] Preparation Example 2: Synthesis of 1-benzyl-1H-indole-6-carbaldehyde
[0468] 1-H-Indole-6-carbaldehyde (320 mg, 2.2 mmol) and benzyl bromide (0.36 ml, 3.3 mmol) were reacted according to the method of Preparative Example 1 to obtain the title compound (400 mg, 77%).
[0469] NMR: 1 H-NMR (400 HMz, CDCl 3 ); δ10.06(s, 1H), 7.86(s, 1H), 7.73(d 1H), 7.64(dd, 1H), 7.35(d, 1H), 7.28-7.33(m, 3H), 7.12(dd , 2H), 6.62(d, 1H), 5.41(s, 2H)
preparation Embodiment 3
[0470] Preparation Example 3: Synthesis of 1-benzyl-3-chloro-1H-indole-6-carbaldehyde
[0471] 1-Benzyl-1H-indole-6-carbaldehyde (835 mg, 3.5 mmol) from Preparative Example 2 was dissolved in tetrahydrofuran (30 ml). N-chlorosuccinimide (NCS, 474 mg, 3.5 mmol) was added dropwise thereto, and the mixture was stirred at 70°C for 2 hours. After the reaction was completed, water was added thereto, and the mixture was extracted with ethyl acetate. The extract was washed with saturated aqueous sodium chloride solution, dried over anhydrous magnesium sulfate, and filtered. The filtrate was distilled under reduced pressure and separated by column chromatography to obtain the title compound (800 mg, 76%).
[0472] NMR: 1 H-NMR (400 HMz, CDCl 3 ); δ10.29(s, 1H), 7.92(s, 1H), 7.79(m, 2H), 7.37(m, 4H), 7.20(d, 2H), 5.41(s, 2H)
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com