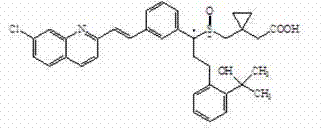
Pharmaceutical composition containing montelukast sodium
A technology of montelukast sodium and composition, applied in the field of medicine, can solve the problems of easy discoloration and decomposition when exposed to light, poor formulation stability, no improvement in tablet stability, etc., and achieves simple and easy production operation and good stability. Effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
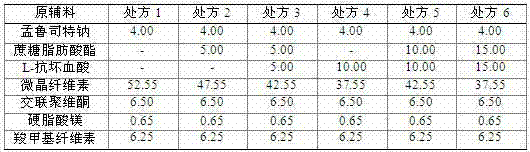
[0063] prescription
[0064]
[0065] Preparation
[0066] (1) Mix and pulverize sucrose fatty acid ester and L-ascorbic acid through 80 mesh to obtain mixed powder;
[0067] (2) Spray the absolute ethanol solution of montelukast sodium onto the starch;
[0068] (3) Granulating the mixed powder obtained in steps (1) and (2) with dry starch and carboxymethyl cellulose;
[0069] (4) Mix the granules obtained in step (3) with micropowder silica gel, pack separately, and obtain.
Embodiment 2
[0071] prescription
[0072]
[0073] Preparation
[0074] (1) Mix and pulverize sucrose fatty acid ester and L-ascorbic acid through 80 mesh to obtain mixed powder;
[0075] (2) Spray the ethanol solution of montelukast sodium onto the lactose;
[0076] (3) Granulating the mixed powder obtained in steps (1) and (2) with sodium carboxymethyl starch and carboxymethyl cellulose;
[0077] (4) Mix the granules obtained in step (3) with talcum powder, pack separately, and obtain.
Embodiment 3
[0079] prescription
[0080]
[0081] Preparation
[0082] (1) Mix and pulverize sucrose fatty acid ester and L-ascorbic acid through 80 mesh to obtain mixed powder;
[0083] (2) Spray the absolute ethanol solution of montelukast sodium on the powdered sugar;
[0084] (3) Granulating the mixed powder obtained in steps (1) and (2) with low-substituted hydroxypropyl cellulose and carboxymethyl cellulose;
[0085] (4) Mix the granules obtained in step (3) with micropowder silica gel, pack separately, and obtain.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com