Medicine composition for treating primary alveolar hydatid disease as well as preparation method and application thereof
A technology of alveolar echinococcosis and composition, applied in the field of medicine, capable of solving problems such as interruption of treatment
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0017] Embodiment 1: the preparation of Chinese medicine dry plaster powder
[0018] (1) Weigh 1.2kg of Altairum chinensis and 1.2kg of Shijunzi, combine them, crush them, and extract 3 times with an ethanol solution with a volume fraction of 95%. Refluxing extraction with an ethanol solution about 10 times the total amount of secondary medicinal materials for 1.5 hours, filtering through four layers of gauze, and combining the ethanol extract;
[0019] (2) Weigh 0.3kg Daphne genkwa, 0.9kg cassia twig, 0.9kg green bark, 1.2kg black plum, 0.9kg Atractylodes macrocephala, and 1.1kg Trichosanthes bark, combine, crush, decoct twice with water, and add the total amount of medicinal materials for the first time About 10 times of purified water, decoct for 1.5 hours, add purified water of about 8 times the total amount of medicinal materials for the second time, decoct for 1 hour, and combine the decoction;
[0020] (3) Concentrate the decoction in step (2) under reduced pressure at...
Embodiment 2
[0021] Example 2: Effect test of traditional Chinese medicine dry ointment powder on rats with primary alveolar echinococcosis
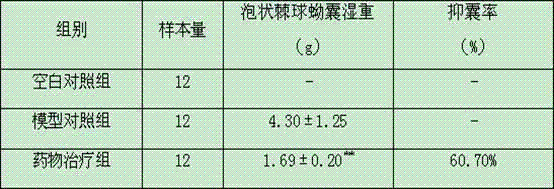
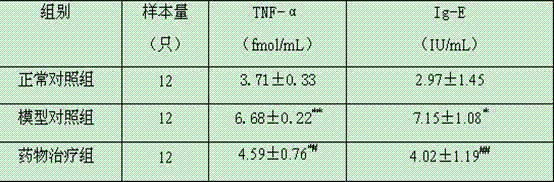
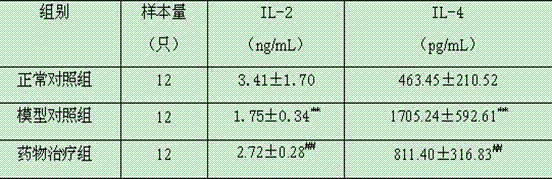
[0022] Kunming female mice, weighing 18-22g. Twelve mice were randomly selected as the blank control group, and the remaining mice were intragastrically administered 100 mL alveolar Echinococcus eggs (storage concentration: 2000 eggs / 1 mL PBS) to establish a primary alveolar echinococcosis infection model. The model mice were randomly divided into drug treatment group and model control group, 12 in each group. The primary alveolar coccus infection mouse model was given 2 months after the establishment of the drug, and the test drug dosage standard was as follows: taking the body weight of the mice as a benchmark, intragastric administration of 618.2 mg / kg of Chinese medicine dry cream powder prepared in Example 1, 1 time / d, continuous treatment for 35 days. Both the model control group and the blank control group were given the same volume of disti...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com