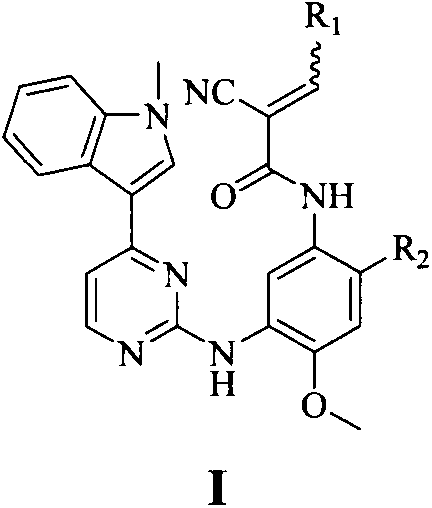
Miazines compound, preparation method and medical application thereof
A technology of pyrimidines and compounds, which can be used in pharmaceutical formulations, organic chemistry, anti-tumor drugs, etc., and can solve off-target problems
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
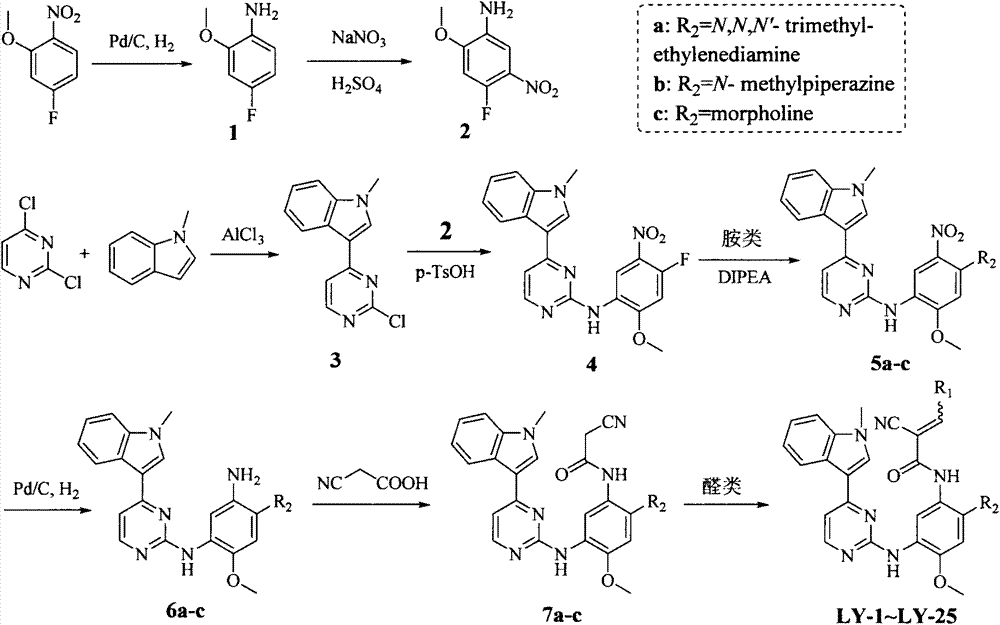
Embodiment 1
[0054]
[0055] Preparation of 2-methoxy-4-fluoroaniline (1)
[0056] Add 2-nitro-5-fluoroanisole (1.00g, 5.81mmol) and 5% palladium carbon (0.62g, 0.29mmol) into 35mL THF, stir with hydrogen gas at room temperature for 5h, filter with suction, and spin to dry the solvent , 0.80 g of a light yellow solid was obtained, with a yield of 97%.
[0057] Preparation of 2-methoxy-4-fluoro-5-nitroaniline (2)
[0058] Under ice bath, 1 (1.00g, 5.42mmol) was dissolved in 6.50mL concentrated sulfuric acid in batches, and NaNO was added in batches 3 (0.46g, 5.41mmol), continued to stir for 4 hours, adjusted to neutral with 2% NaOH solution, extracted with dichloromethane, and spin-dried the solvent to obtain 0.64g of an orange-red solid, with a yield of 64%.
[0059] Preparation of 1-methyl-3-(2-chloropyrimidin-4-yl)indole (3)
[0060] Dissolve 2,4-dichloropyrimidine (1.00 g, 6.71 mmol) in 25 mL of ethylene glycol dimethyl ether, cool in an ice bath, and add AlCl in batches 3 (0.98g...
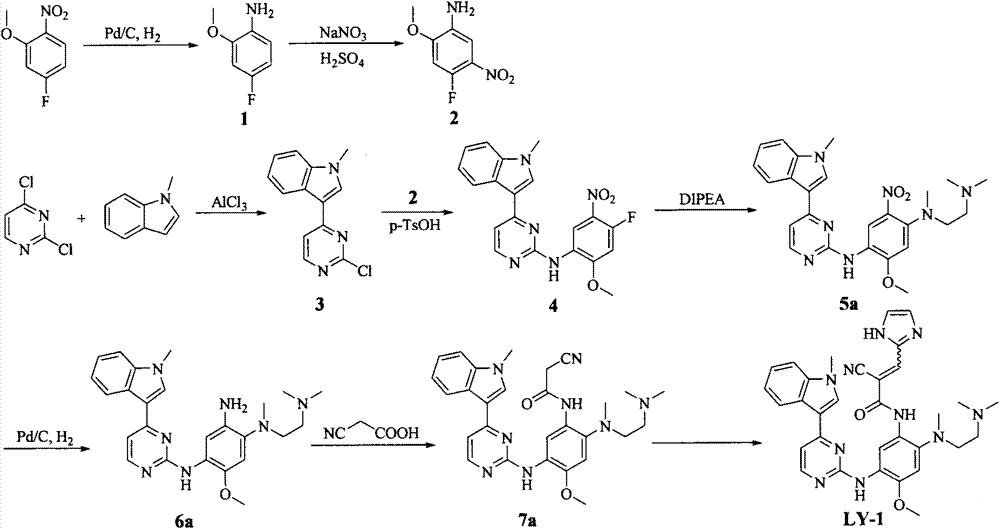
Embodiment 2
[0072] N-[2-[[2-(Dimethylamino)ethyl](methyl)amino]-4-methoxy-5-[[4-(1-methylindol-3-yl) Preparation of pyrimidin-2-yl]amino]phenyl]-2-cyano-3-(imidazol-2-yl)acrylamide (LY-2)
[0073]
[0074] Referring to the preparation method of LY-1, a yellow powdery solid was obtained by reacting 7a with imidazole-2-carbaldehyde, the yield was 73%, and mp: 137-139°C. ESI-MS: 591.4[M+H] + ; 1 H-NMR (300MHz, DMSO-d 6 ), δ(ppm): 2.11(s, 6H), 2.28(br, 2H), 2.72(s, 3H), 2.99(br, 2H), 3.88(s, 3H), 3.90(s, 3H), 7.14 (br, 2H), 7.24(br, 2H), 8.06(br, 2H), 8.26(d, 1H, J=6.93Hz), 8.34(m, 1H), 8.59(s, 1H), 9.19(s, 1H), 10.24(br, 1H).
Embodiment 3
[0076] N-[2-[[2-(Dimethylamino)ethyl](methyl)amino]-4-methoxy-5-[[4-(1-methylindol-3-yl) Preparation of pyrimidin-2-yl]amino]phenyl]-2-cyano-3-(furan-2-yl)acrylamide (LY-3)
[0077]
[0078] Referring to the preparation method of LY-1, a yellow powdery solid was obtained by reacting 7a with furan-2-carbaldehyde, the yield was 81%, and mp: 174-176°C. ESI-MS: 591.4[M+H] + ; 1 H-NMR (300MHz, DMSO-d 6 ), δ(ppm): 2.11(s, 6H), 2.45(m, 2H), 2.72(s, 3H), 2.99(m, 2H), 3.88(s, 3H), 3.91(s, 3H), 6.87 (m, 1H), 7.14(m, 2H), 7.24(m, 2H), 7.46(d, 1H, J=3.54Hz), 7.52(d, 1H, J=7.98Hz), 7.99(s, 1H) , 8.08(s, 1H), 8.21(s, 1H), 8.26(d, 1H, J=8.10Hz), 8.33(d, 1H, J=5.31Hz), 8.60(s, 1H), 9.16(s, 1H), 10.26(s, 1H).
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com