Copper metal complex and compound prepared from copper metal complex and human serum albumin as well as synthesis method and application thereof
A technology of human serum albumin and its synthesis method, which is applied in the field of copper metal complexes and their complexes with human serum albumin, to achieve significant in vitro anti-tumor activity and good medicinal value
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
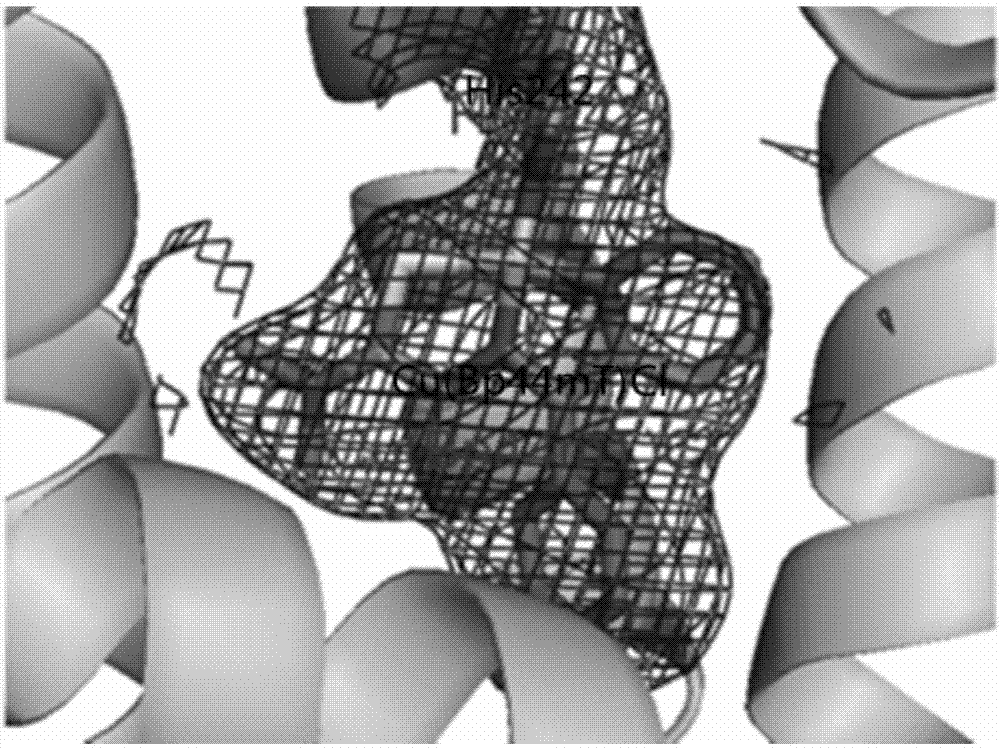
Image
Examples
Embodiment 1
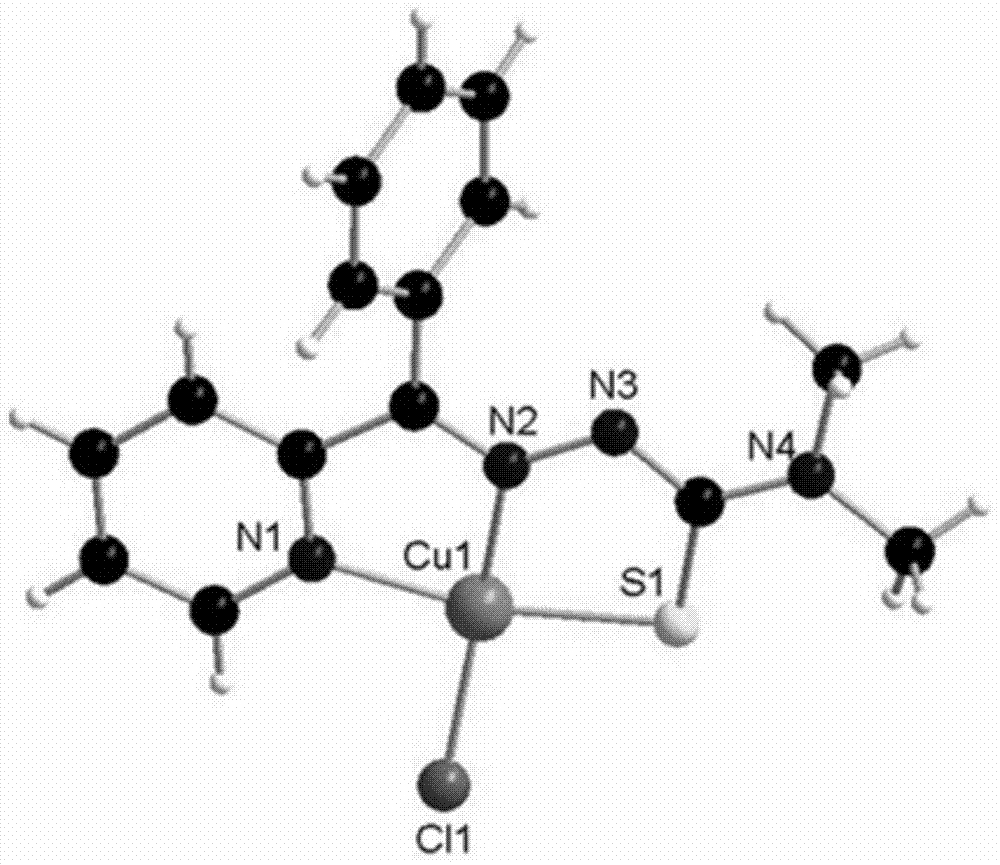
[0033] Embodiment 1: the synthesis of Cu(Bp44mT)Cl complex
[0034] The synthetic route is as follows:
[0035]
[0036] The specific synthesis method is:
[0037]1) Dissolve 10mmol of phenyl-2-pyridyl ketone (1832.1mg) in 20ml of ethanol (the concentration of solvent ethanol is 50v / v%), stir at 50°C for 15min to obtain a solution, and dissolve the above solution one by one Add dropwise to 20ml of ethanol solution (concentration of solvent ethanol: 60v / v%) added with 10mmol 4,4'-dimethyl-3-thiosemicarbazide (1191.9mg), reflux and stir at 50°C for 24h to obtain light yellow For the precipitate, filter the light yellow precipitate obtained above, wash with absolute ethanol and ether three times, and dry to obtain the ligand phenyl-2-pyridyl ketal 4,4'-dimethyl-3-amino Thiourea;
[0038] 2) will contain CuCl 2 2H 2 O (170.48mg, 1mmol) 20ml of methanol (the concentration of solvent methanol is 60v / v%) solution, dropwise added to the solution containing 1mmol of phenyl-2-py...
Embodiment 2
[0051] Embodiment 2: the synthesis of Cu(Bp44mT)Cl complex
[0052] 1) Dissolve 10mmol of phenyl-2-pyridyl ketone (1832.1mg) in 20ml of methanol (the concentration of solvent methanol is 80v / v%), stir at 60°C for 15min to obtain a solution, and dissolve the above solution one by one Add dropwise to 20ml of 10mmol 4,4'-dimethyl-3-thiosemicarbazide (1191.9mg) in methanol (concentration of solvent methanol: 20v / v%) solution, reflux and stir at 50°C for 24h to obtain light yellow For the precipitate, filter the light yellow precipitate obtained above, wash with absolute ethanol and ether three times, and dry to obtain the ligand phenyl-2-pyridyl ketal 4,4'-dimethyl-3-amino Thiourea;
[0053] 2) will contain CuCl 2 2H 2 O (170.48mg, 1mmol) 20ml of methanol (the concentration of solvent methanol is 40v / v%) solution, dropwise added to the solution containing 1mmol phenyl-2-pyridyl ketal 4,4'-dimethyl-3-amino In 20ml of methanol (the concentration of solvent methanol is 30v / v%) so...
Embodiment 3
[0055] Embodiment 3: the synthesis of Cu(Bp44mT)Cl complex
[0056] 1) Dissolve 10mmol of phenyl-2-pyridyl ketone (1832.1mg) in 20ml of ethanol (the concentration of solvent ethanol is 40v / v%), stir at 30°C for 20min to obtain a solution, and dissolve the above solution one by one Add 10mmol 4,4'-dimethyl-3-thiosemicarbazide (1191.9mg) dropwise into 20ml of ethanol (concentration of solvent ethanol: 70v / v%) solution, stir and react at 35°C for 72h to obtain light Yellow precipitate, the light yellow precipitate obtained above was filtered, washed with water three times, and dried to obtain the ligand phenyl-2-pyridyl ketal 4,4'-dimethyl-3-thiosemicarbazide;
[0057] 2) will contain CuCl 2 2H 2 O (170.48mg, 1mmol) 20ml of ethanol (the concentration of solvent ethanol is 80v / v%) solution, dropwise added to the solution containing 1mmol of phenyl-2-pyridyl ketal 4,4'-dimethyl-3-amino In 20ml of ethanol (the concentration of solvent ethanol is 50v / v%) solution of thiourea ligan...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com