Monohydrate crystal of fimasartan potassium salt, its preparation method and pharmaceutical composition containing it
A technology of fimasartan and monohydrate, which is applied in the direction of drug combinations, organic chemical methods, and medical preparations containing active ingredients, can solve the problems of increased processing costs, troubles, economic losses, etc., and achieve the goal of reducing processing time Effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
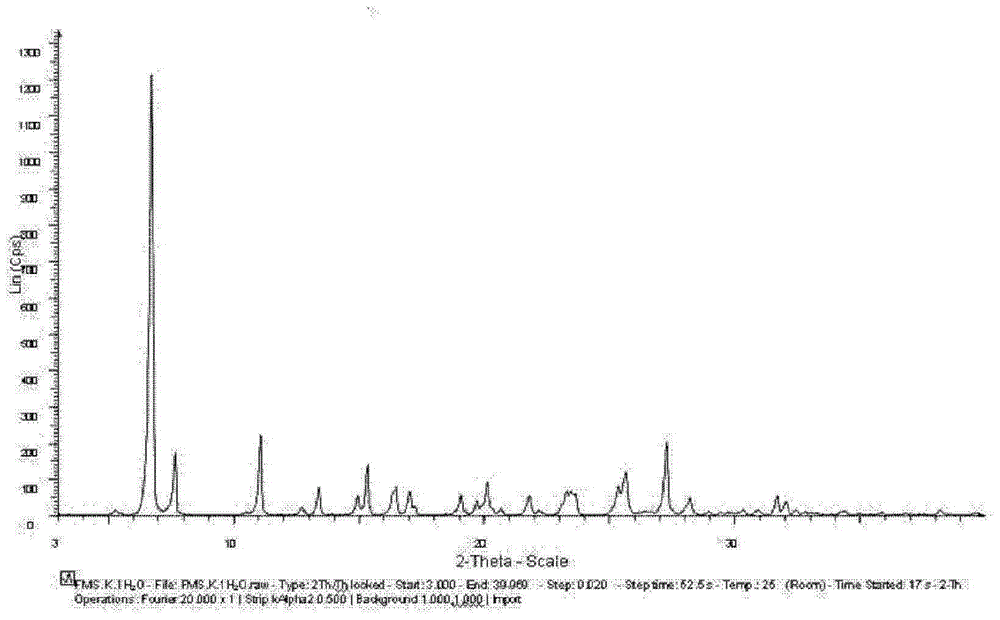
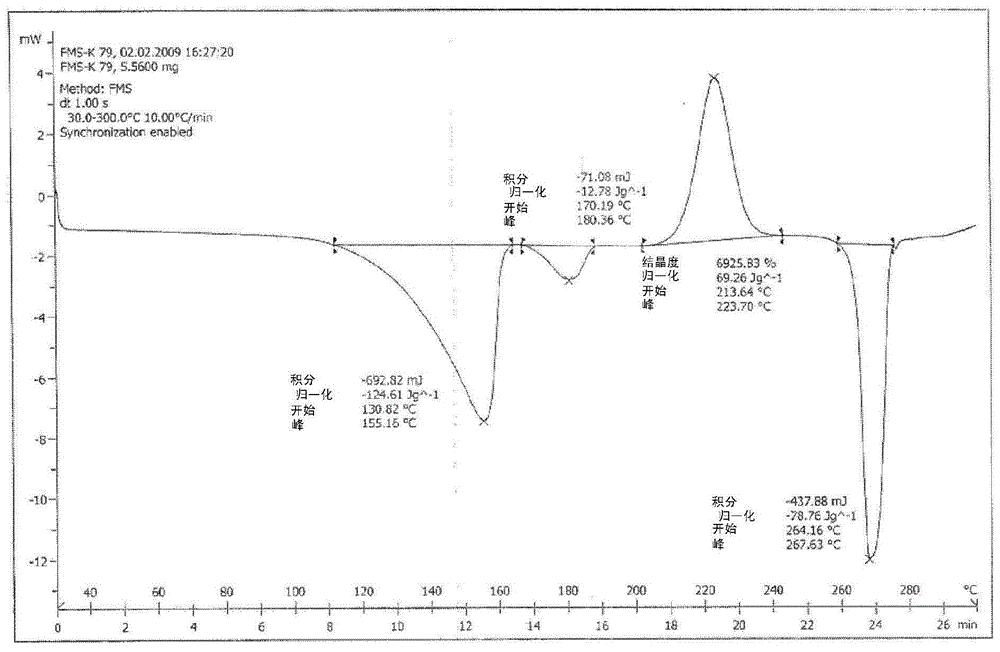
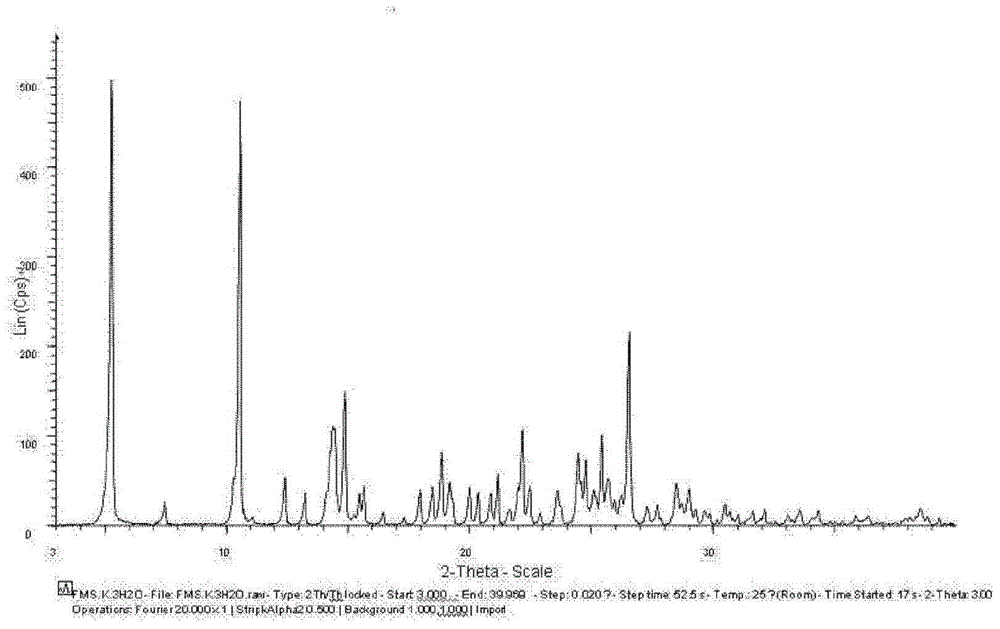
[0115] Embodiment 1: Preparation of Fimasartan potassium salt monohydrate
[0116] By adding 2-n-butyl-5-dimethylaminothiocarbonylmethyl-6-methyl-3-[[2'-(1H-tetrazol-5-yl) to isopropanol (18.5 mL) Biphenyl-4-yl]methyl]pyrimidin-4(3H)-one (hereinafter referred to as Fimasartan) (6.55 g, 12.6 mmol) was used to prepare a suspension.
[0117] To the prepared suspension was added a solution prepared by mixing isopropanol (19.6 mL), potassium hydroxide (1.18 g, 21.0 mmol) and 2-ethylhexanoic acid (2.83 g, 19.6 mmol), dissolved at 81°C , then stirred at reflux for 1 hour. Then, the mixture was cooled to 25°C to precipitate crystals, and the obtained solid was filtered and washed successively with isopropanol (2.5 mL) and ethyl acetate (2.0 mL).
[0118] Then, the crystals were dried at 40° C. for 8 hours under a reduced pressure of 10 mmHg to yield fimasartan potassium salt monohydrate in the form of a white powder solid.
[0119] Yield: 6.49g (95.5%)
[0120] Moisture content (KF ...
Embodiment 2
[0121] Embodiment 2: Preparation of Fimasartan potassium salt monohydrate
[0122] A suspension was prepared by adding Fimasartan (8.52 g, 16.4 mmol) to isopropanol (18.5 mL).
[0123] To the prepared suspension was added a solution prepared by mixing isopropanol (19.6 mL), potassium hydroxide (1.18 g, 21.0 mmol) and 2-ethylhexanoic acid (2.83 g, 19.6 mmol), dissolved at 81°C , then stirred at reflux for 1 hour. Then, the mixture was cooled to 25°C to precipitate crystals, and the obtained solid was filtered and washed with isopropanol (2.5 mL) and ethyl acetate (2.0 mL).
[0124] Then, the crystals were dried at 40° C. for 8 hours under a reduced pressure of 10 mmHg to yield fimasartan potassium salt monohydrate in the form of a white powder solid.
[0125] Yield: 8.35g (94.4%)
[0126] Moisture content (KF method): 3.70%
Embodiment 3
[0127] Embodiment 3: Preparation of Fimasartan potassium salt monohydrate
[0128] A suspension was prepared by adding Fimasartan (8.91 g, 17.1 mmol) to isopropanol (18.5 mL).
[0129] To the prepared suspension was added a solution prepared by mixing isopropanol (19.6 mL), potassium hydroxide (1.18 g, 21.0 mmol) and 2-ethylhexanoic acid (2.83 g, 19.6 mmol), dissolved at 81°C , then stirred at reflux for 1 hour. Then, the mixture was cooled to 25°C to precipitate crystals, and the obtained solid was filtered and washed with isopropanol (2.5 mL) and ethyl acetate (2.0 mL).
[0130] Then, the crystals were dried at 40° C. for 8 hours under a reduced pressure of 10 mmHg to yield fimasartan potassium salt monohydrate in the form of a white powder solid.
[0131] Yield: 8.65g (93.8%)
[0132] Moisture content (KF method): 3.44%
PUM
| Property | Measurement | Unit |
|---|---|---|
| melting point | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com