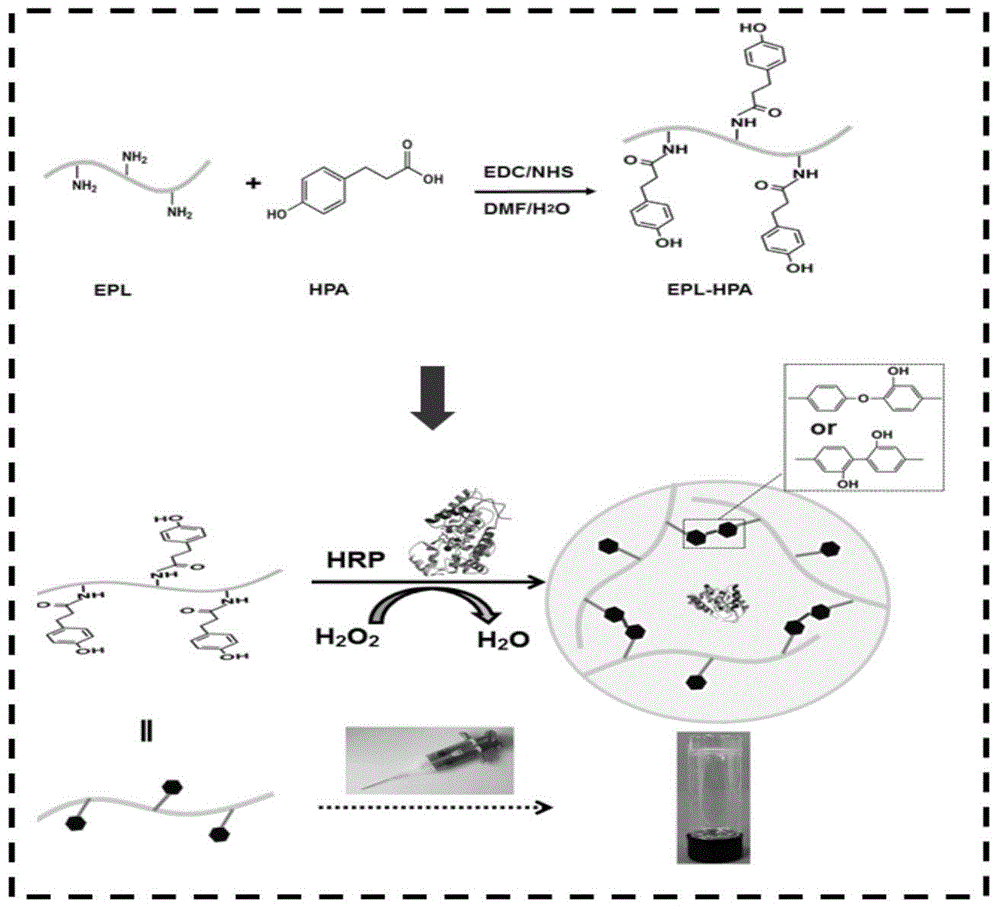
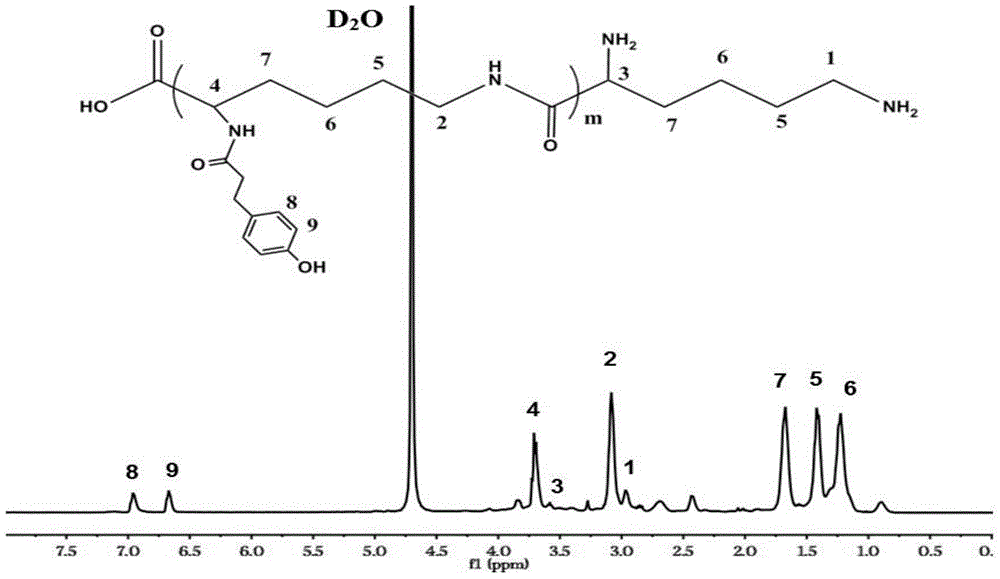
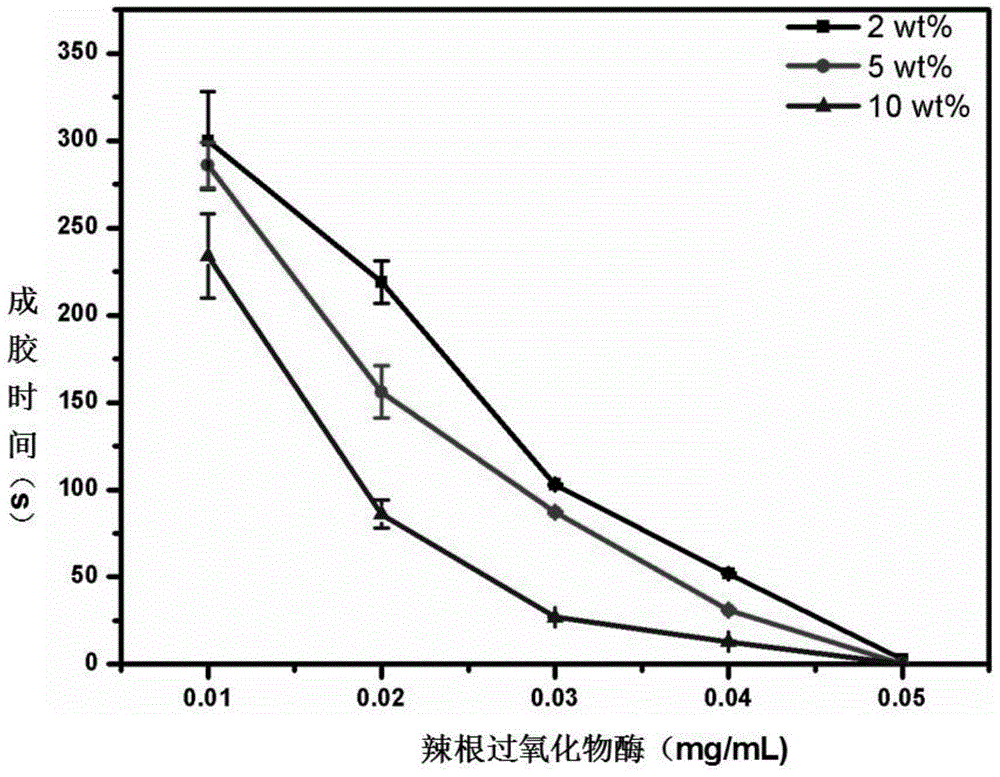
Epsilon-polylysine-p-hydroxybenzene propanoic acid antibiotic hydrogel dressing and preparation method thereof
A technology of p-hydroxyphenylpropionic acid and polylysine, which is applied in the field of medical materials, can solve the problems of limited use, low mechanical strength, poor biocompatibility, etc., and achieve excellent biocompatibility and biodegradability Sexuality, prevention of wound infection, excellent inhibitory effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0050] Dissolve p-hydroxyphenylpropionic acid in a blend solvent of N,N-dimethylformamide (DMF) and deionized water (RO water). The volume ratio of DMF to RO water is 1:3. The dosage of the acid is 1g / 100mL of the blending solvent, stirring and mixing evenly. Add 1-(3-dimethylaminopropyl)-3-ethylcarbodiimide hydrochloride (EDC) and N-hydroxysuccinimide (NHS), and activate it under ice-bath conditions for 5h. Add ε-polylysine dissolved in RO water into the activated system, and react for 15 hours at room temperature. The molar ratio of each substance is as follows: EPL (NH 2 ):HPA=1:1, EDC:HPA=1:1, EDC:NHS=1:1. The reacted solution was transferred to a dialysis bag and dialyzed in RO water for 5 days. The obtained purified solution after dialysis was freeze-dried to obtain EPL-HPA copolymer with a yield of 45%.
Embodiment 2
[0052] Dissolve p-hydroxyphenylpropionic acid in a blend solvent of N,N-dimethylformamide (DMF) and deionized water (RO water). The volume ratio of DMF to RO water is 1:3. The dosage of the acid is 1g / 100mL of the blending solvent, stirring and mixing evenly. Add 1-(3-dimethylaminopropyl)-3-ethylcarbodiimide hydrochloride (EDC) and N-hydroxysuccinimide (NHS), and activate it under ice-bath conditions for 5h. Add polylysine dissolved in RO water into the activated system, and react for 15 hours at room temperature. The molar ratio of each substance is as follows: EPL (NH 2 ):HPA=1:3, EDC:HPA=1:1, EDC:NHS=1:1. The reacted solution was transferred to a dialysis bag and dialyzed in RO water for 5 days. The obtained purified solution after dialysis was freeze-dried to obtain EPL-HPA copolymer with a yield of 87%.
Embodiment 3
[0054] Dissolve p-hydroxyphenylpropionic acid in a blend solvent of N,N-dimethylformamide (DMF) and deionized water (RO water). The volume ratio of DMF to RO water is 1:3. The dosage of the acid is 1g / 100mL of the blending solvent, stirring and mixing evenly. Add 1-(3-dimethylaminopropyl)-3-ethylcarbodiimide hydrochloride (EDC) and N-hydroxysuccinimide (NHS), and activate it under ice-bath conditions for 5h. Add polylysine dissolved in RO water into the activated system, and react for 15 hours at room temperature. The molar ratio of each substance is as follows: EPL (NH 2 ):HPA=1:5, EDC:HPA=1:1, EDC:NHS=1:1. The reacted solution was transferred to a dialysis bag and dialyzed in RO water for 5 days. The obtained purified solution after dialysis was freeze-dried to obtain EPL-HPA copolymer with a yield of 63%.
PUM
| Property | Measurement | Unit |
|---|---|---|
| Elastic modulus | aaaaa | aaaaa |
| Elastic modulus | aaaaa | aaaaa |
| Elastic modulus | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com