New crystal form B of Betahistine mesilate and preparation method therefor
A technology of methanesulfonic acid and stin crystal, applied in the field of medicine, can solve the problems of strong hygroscopicity and difficult preparation, and achieve the effects of good stability, good reproducibility and mild reaction conditions
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
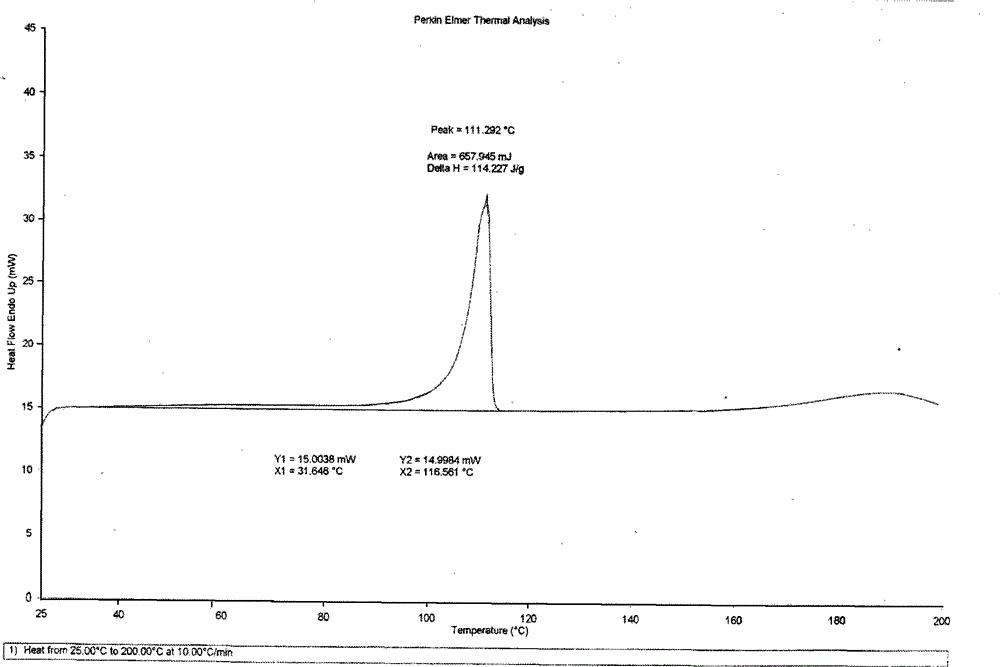
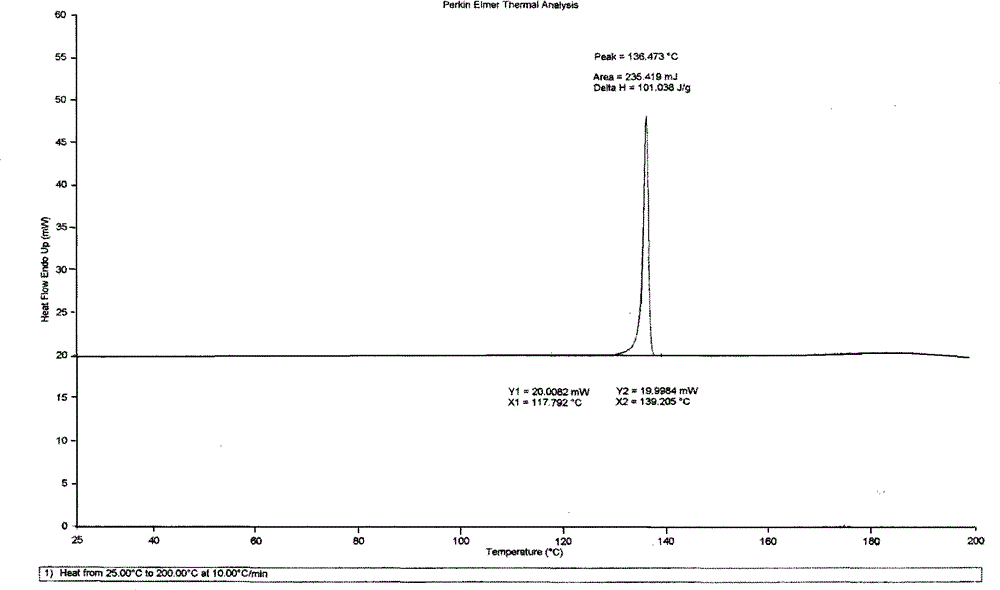
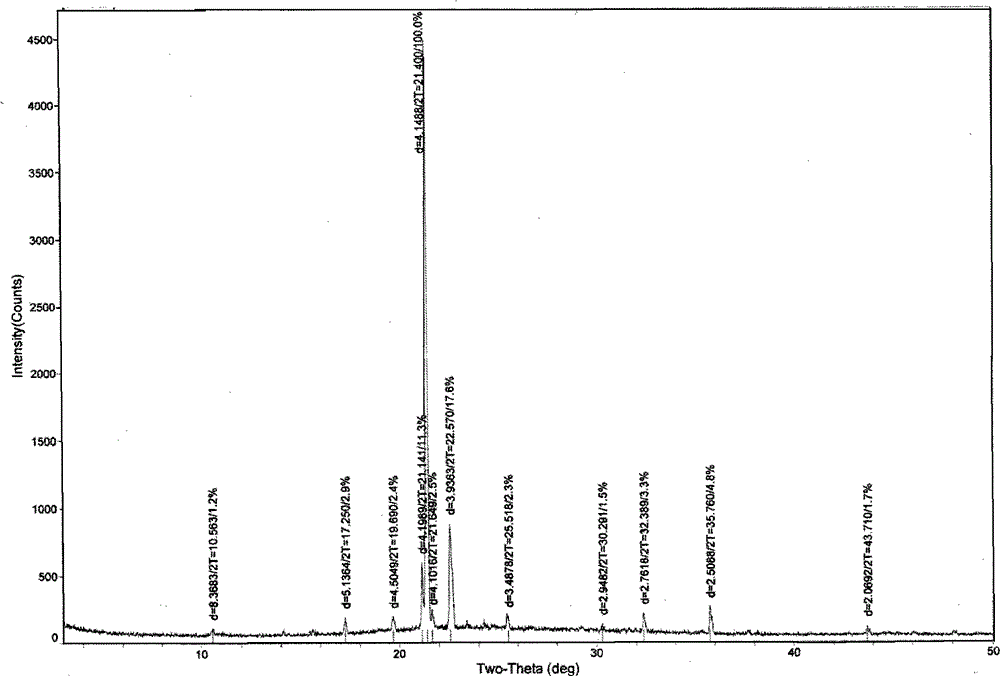
[0037] Put 10g of betahistine mesylate into 100mL of isopropanol, add 2mL of water, heat up to 50°C under stirring, dissolve all the solids, keep stirring for 30min, cool down to 30°C and stir for 3h to slowly crystallize, filter, The filter cake was air-dried at 45±5°C to obtain 6.6 g of white crystals of betahistine mesylate, whose melting point was 136-138°C according to differential scanning calorimetry (DSC). figure 2 The main test data of its X-ray diffraction is shown in the table below (listing the test data with relative intensity greater than 2%), and the accompanying drawings are shown in the attached description. Figure 4 .
[0038]
Embodiment 2
[0040] Put 10g of betahistine mesylate into 75mL of ethanol, add 1mL of water, heat up to 50°C under stirring, dissolve all the solids, keep stirring for 30min, cool down to 0°C and stir for 3h to crystallize, filter, and filter cake in Blow drying at 45±5°C to obtain 4.2 g of white crystals of betahistine mesylate. The differential scanning thermogram (DSC chart) and X-ray diffraction chart are respectively attached to the instructions figure 2 , Figure 4 Consistent within the margin of error.
[0041] implementation
[0042] Put 10g of betahistine mesylate into 150mL of tetrahydrofuran, add 3mL of water, heat up to 50°C under stirring to dissolve all the solids, keep stirring for 30min, cool down to 0°C and stir for 3h to slowly crystallize, filter, and the filter cake is Blow drying at 45±5°C to obtain 3.7 g of white crystals of betahistine mesylate B. The differential scanning thermogram (DSC chart) and X-ray diffraction chart are respectively attached to the instruc...
Embodiment 4
[0044]Put 10g of betahistine mesylate into 100mL of a mixed solution of ethylene glycol and acetonitrile (50:50), add 1mL of water, heat up to 55°C with stirring to dissolve all the solids, keep stirring for 30min, and cool down to 30°C Slowly crystallized, filtered, and the filter cake was air-dried at 45±5°C to obtain 3.3 g of white crystals of betahistine mesylate B. The differential scanning thermogram (DSC chart) and X-ray diffraction chart are respectively attached to the instructions figure 2 , Figure 4 Consistent within the margin of error.
PUM
| Property | Measurement | Unit |
|---|---|---|
| melting point | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com