New application of multi-poly ADP RNA polymerase inhibitor in treating HBV-related diseases
A hepatitis B virus and inhibitor technology, applied in the treatment of diseases related to hepatitis B virus infection, phthalazinone compounds for the treatment or prevention of diseases related to hepatitis B virus infection, liver cancer field
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
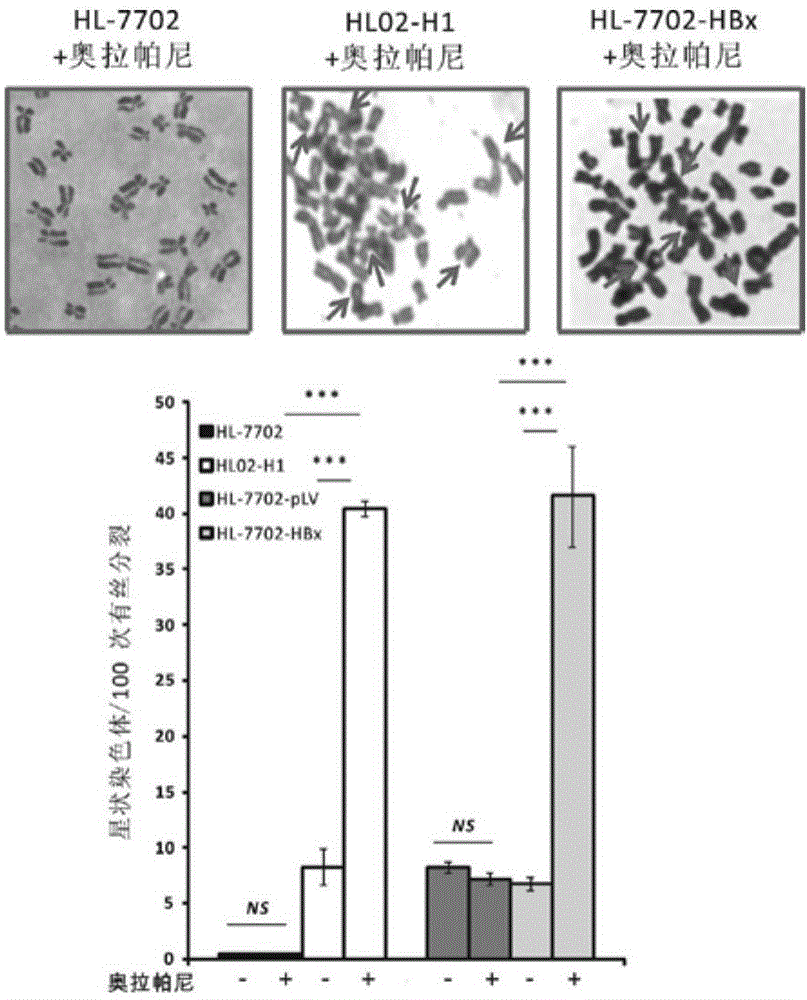
[0048] Example 1 Olaparib increases the probability of chromosome breakage in HL02-H1 cells.
[0049] 1. Hepatitis B virus (HBV) genome-wide transgenic human liver cell line HL02-H1
[0050] HL-7702 cells were cultured in 5cm×5cm culture flasks until the density reached 90%. After trypsinization, re-spread to 6cm petri dishes for a total of 3 dishes and culture overnight. Transfect 10 μg of pcDNA3.1-HBV-wholeGenome plasmid into each plate of cells, and the transfection process is as follows: Add 10 μg of plasmid and TurboFect transfection reagent (Thermo, #R0531) into Opti-MEM serum-free medium, mix well, and let stand for 20 minutes. The mixture was added to the overnight cultured cells, and G418 was added 48 hours later for selection (final concentration 800 μg / ml). Continue to screen for one month, digest the surviving cells, dilute to 4 cells / ml with complete medium, add the diluted cell culture to a 96-well plate, 200 μl per well, to ensure that each well in the 96-well...
Embodiment 2
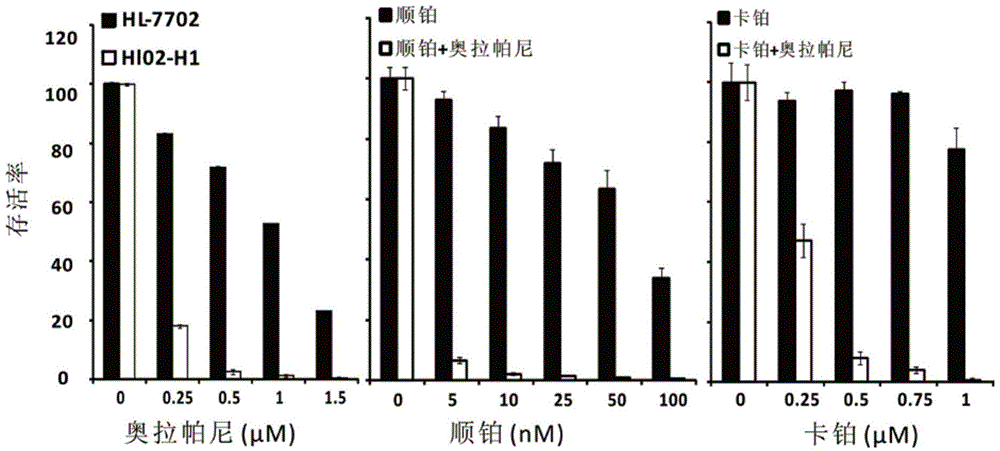
[0056] Example 2 Olaparib significantly inhibits the proliferation of hepatitis B virus genome-wide transgenic human liver cell line HL02-H1
[0057] In order to further confirm the inhibitory effect of olaparib on the proliferation of HBV-positive cells, the inhibitory effect of olaparib on HBx-positive malignant liver cells HL02-H1 was tested by colony formation assay.
[0058] Cells HL-7702 as the control group and HBV-positive HL02-H1 cells were inoculated into 10 cm diameter culture dishes, and drugs or the same volume of DMSO were added as controls after the cells adhered to the wall. 37°C, 5% CO 2 After culturing for 10 days, the cells were fixed with methanol, stained according to the instructions of the Giemsa stain kit, and the number of clones was counted. The total number of plates required is determined according to the concentration of the drug gradient. The final concentration of olaparib single drug is 0, 0.25, 0.5, 1, 1.5 μM, the dose of cisplatin combined wit...
Embodiment 3
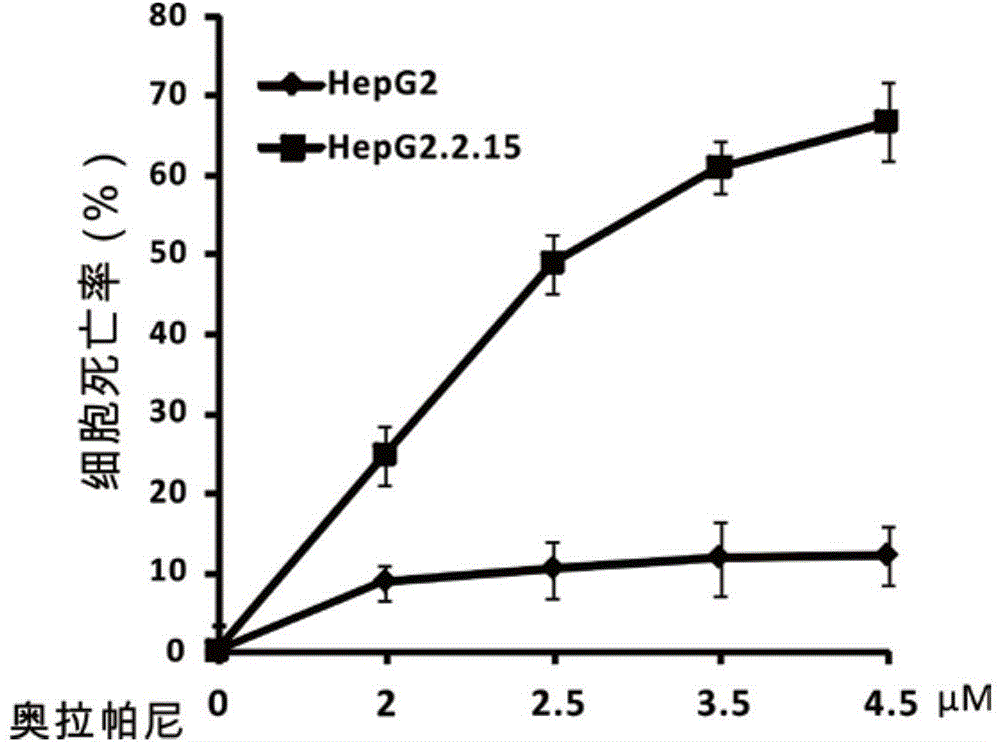
[0060] Example 3. The killing effect of olaparib on hepatitis B virus-positive liver cancer cells (HepG2.2.15) Experimental method: collect logarithmic phase liver cancer cells HepG2 and HepG2.2.15 cells, use a cell counting board to count, adjust the concentration of the cell suspension, The two kinds of cells were respectively added to two 96-well plates, 100ul was added to each well, and the cell density was adjusted to 1000 cells / well, and the edge wells were filled with sterile PBS. Incubate at 37°C in 5% CO2 until the cell monolayer covers the bottom of the well, then add gradient concentrations of olaparib so that the final drug concentration in each well is 0, 2, 2.5, 3.5, 4.5 μM. Three replicate wells were set up for each well. At the same time set the zero well (medium, MTT, dimethyl sulfoxide). After incubating for 16-48 hours under the condition of 5% CO2 and 37° C., 20 μl of MTT solution (5 mg / ml, ie 0.5% MTT) was added to each well, and the culture was continued...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com