Novel purification processing process for R-9-(2-hydroxypropyl)adenine
An R-9-, hydroxypropyl technology, applied in the field of organic synthesis, can solve the problems of increasing the difficulty of separation, waste of raw materials, difficult separation of isomers, etc., and achieves the effect of high practical value and reduced separation cost.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
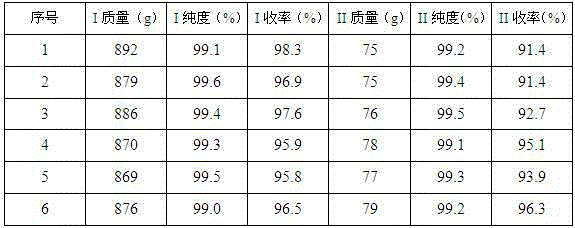
Examples
Embodiment 1
[0024] Step 1: Obtain R-9-(2-hydroxypropyl)adenine crude product
[0025] Add 100 g of adenine (0.74 mol) and 2.35 g (0.058 mol) of sodium hydroxide to 500 ml of DMF respectively, stir at room temperature for 20 min, add 96.5 g (0.946 mol) of R-propylene carbonate, and raise the temperature to 120 ~130℃ heat preservation reaction for 16 hours;
[0026] After the reaction was detected by HPLC, steam the solution of R-9-(2-hydroxypropyl)adenine under reduced pressure to remove about 70-80% of the DMF solvent, add 300ml of toluene, stir evenly, and keep warm at 0°C for 2 h, filtered, and dried to obtain 136 g of R-9-(2-hydroxypropyl)adenine crude product, with a crude yield of 95.7%, a purity of 90.8%, and an isomer of 8.0%.
[0027] Step 2: Column packing and pretreatment
[0028] Weigh one 150g of PRP-6A resin, pour it into a glass chromatography column, wash it with ethanol repeatedly 3 times to make the column compact and free of air bubbles, the resin height is 28c...
Embodiment 2
[0042] Step 1: Obtain R-9-(2-hydroxypropyl)adenine crude product
[0043] The amount of adenine was changed to 500 g, and the operation was the same as step 1 of Example 1 to obtain 675 g of R-9-(2-hydroxypropyl) adenine crude product, with a crude product yield of 95.1%, a purity of 91.9%, and an isomer of 7.8%.
[0044] Step 2: Column packing and pretreatment
[0045] Weigh 1.5kg of PRP-6A resin, pour it into a glass chromatography column, wash it repeatedly with methanol 3 times to make the column compact and free of air bubbles, the height of the resin is 50 cm, and the column is uniform, then wash it repeatedly with ethyl acetate 3 times , and finally replace ethyl acetate with methanol until no ethyl acetate is tested, and the chromatographic column is ready for use.
[0046] Step 3: Sample loading
[0047] Dissolve 60 g of crude R-9-(2-hydroxypropyl)adenine in a small amount of water (200ml), pour it into the PRP-6A resin chromatographic column, and complete the sampl...
Embodiment 3
[0059] Step 1: Obtain R-9-(2-hydroxypropyl)adenine crude product
[0060] The amount of adenine was changed to 5 kg, and the operation was the same as in step 1 of Example 1 to obtain 6.85 kg of R-9-(2-hydroxypropyl) adenine crude product, with a crude product yield of 96.4%, a purity of 90.7%, and an isomer of 8.2%. .
[0061] Step 2: Column packing and pretreatment
[0062] The quality of PRP-6A resin is 25 kg. The operation procedure is the same as that of step 2 of Example 1.
[0063] Step 3: Sample loading
[0064] The amount of sample loaded was 1 kg, and the operation was the same as Step 3 of Example 1.
[0065] Step 4: Elution
[0066] Prepare 80% ethanol water as the eluent, pour it into the resin chromatographic column, use the air pump to pressurize the elution, the flow rate is controlled at 150ml / min, use the receiving bottle to receive the eluent every 500ml in turn, and HPLC detects the product in each bottle content.
[0067] Step Five: Concentrate ...
PUM
| Property | Measurement | Unit |
|---|---|---|
| purity | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com