Uses of protein precursors as prodrugs
A protein and precursor technology, applied in the field of drug delivery and protein engineering, can solve the problems of final product composition and size heterogeneity
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0040] Expression and Characterization of PI-Tf Recombinant Fusion Protein
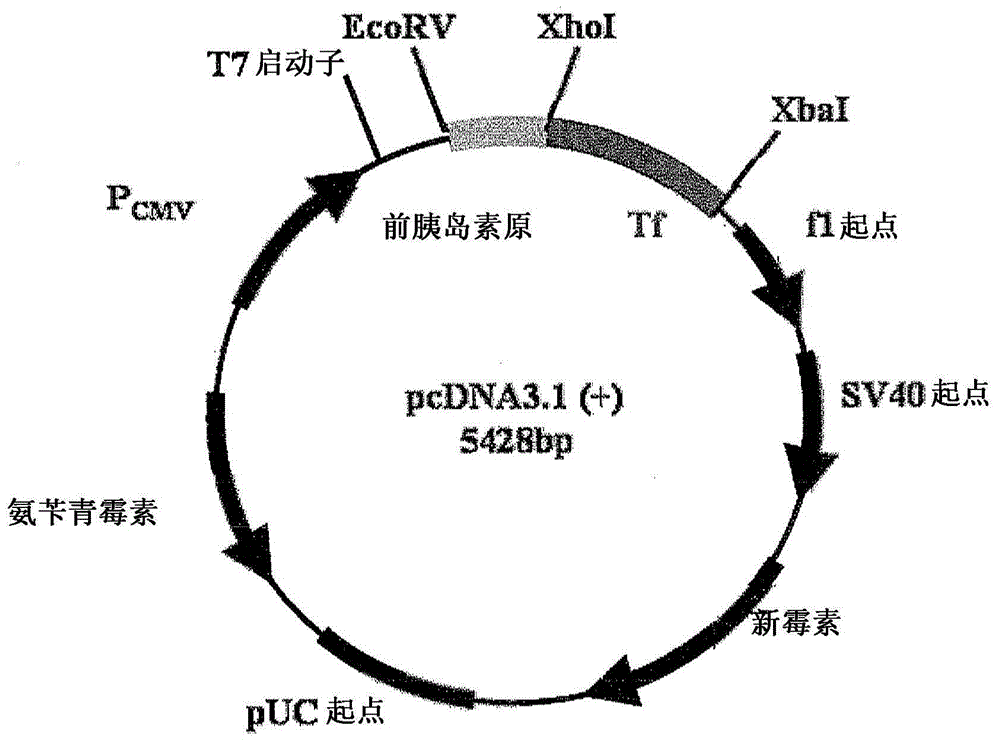
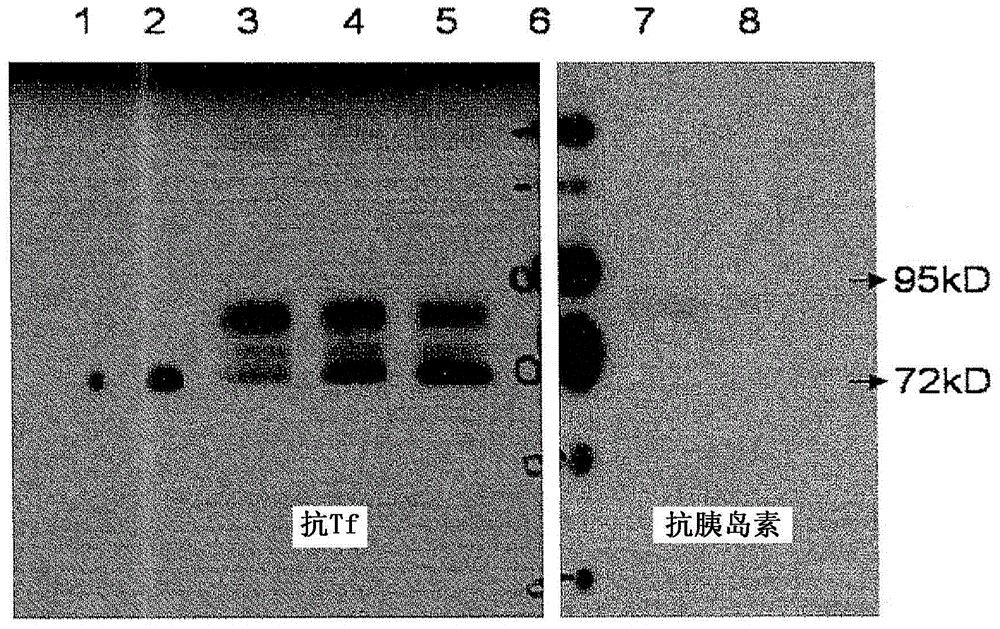
[0041] Using molecular cloning, the preproinsulin sequence (NM_000207) fused in frame with the Tf sequence (NM_001063) was engineered into the pcDNA3.1(+) expression vector (Invitrogen, CA) ( figure 1 ). A plasmid containing the preproinsulin-Tf fusion gene was transiently transfected into HEK 293 cells by polyethyleneimine-mediated DNA transfection. Serum-free conditioned medium was collected, concentrated using a lab-scale Tangential Flow Filtration System (Millipore, MA) and then ultrafiltered using a Centricon (Millipore, MA). The PI-Tf fusion protein was characterized and quantified by Western blotting using anti-Tf antibody (Sigma, MO) and anti-insulin (pro) antibody (Abeam, MA). Anti-Tf and anti-insulin (pro) western blots showed the presence of a major band with a molecular weight of about 89 kD, which indicated that the PI-Tf fusion protein was successfully expressed and secreted into the...
Embodiment 2
[0043] Enhanced inhibitory effect of PI-Tf fusion protein on hepatic glucose production in H4IIE hepatoma cells
[0044] Rat hepatoma H4IIE cells were cultured in high glucose DMEM containing 10% fetal bovine serum. After confluence, cells were treated with different drugs for 24 hours at 37°C. Cells were washed twice with phosphate buffered saline. Glucose production medium consisting of serum-free, glucose-free, phenol red-free DMEM supplemented with 2 mM sodium pyruvate and 40 mM LD-lactate was added to the cells and incubated for an additional 3 hours. The supernatant was harvested and the glucose concentration was measured with the Amplex Red Glucose / Glucose Oxidase Kit (Invitrogen, CA) [5]. Cells were lysed in 1M NaOH, and protein amounts were determined with BCA (Thermo Scientific, IL).
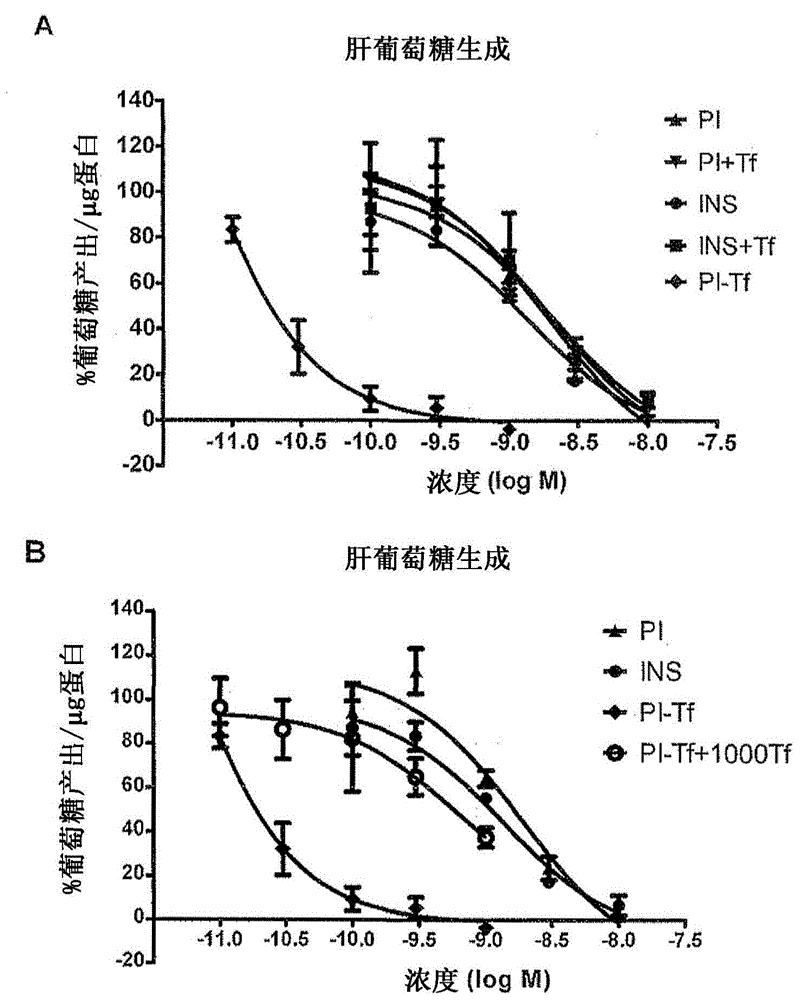
[0045] Proinsulin and insulin showed comparable inhibitory effects on glucose production, with IC 50 The values were 1441.3±641.6 pM and 1093.9±105.6 pM, respectively (Fig. 3A)...
Embodiment 3
[0047] Liver cancer cells convert PI-Tf to insulin-Tf fusion protein
[0048] Rat hepatoma H4IIE cells (ATCC, VA) were treated with PI-Tf fusion protein in DMEM medium and incubated at 37°C. Media were collected at various times and subjected to insulin-specific and proinsulin-specific radioimmunoassays (Millipore, MA). Concentrations of proinsulin and insulin were obtained based on standard curves from radioimmunoassays. After up to 24 hours of treatment in H4IIE cells, insulin-containing material was continuously produced in PI-Tf fusion protein-treated samples, but not in proinsulin-treated samples ( Figure 4 ). The resulting insulin-containing material was shown to be insulin-Tf rather than released insulin because the two moieties are linked by a stable peptide bond. The conversion efficiency of PI-Tf to insulin-Tf was estimated to be 8.8% when the dose of PI-Tf was 10 nM, and 21.6% when the dose of PI-Tf was 1 nM. These results demonstrate that the prohormone fusi...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com