Application of compound in preparation of osteoarthritis therapeutic drug
A technology for osteoarthritis and compounds, applied in the field of medicine, can solve the problems of limited promotion, high cost and time cost, long onset time, etc., and achieve the effects of improving the quality of life, easy dosage, and dosage control
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0026] 1. Take out the cartilage in the joints of the extremities of the 24-hour-old SD rats, and extract the chondrocytes. Passage the first passage cells for study.
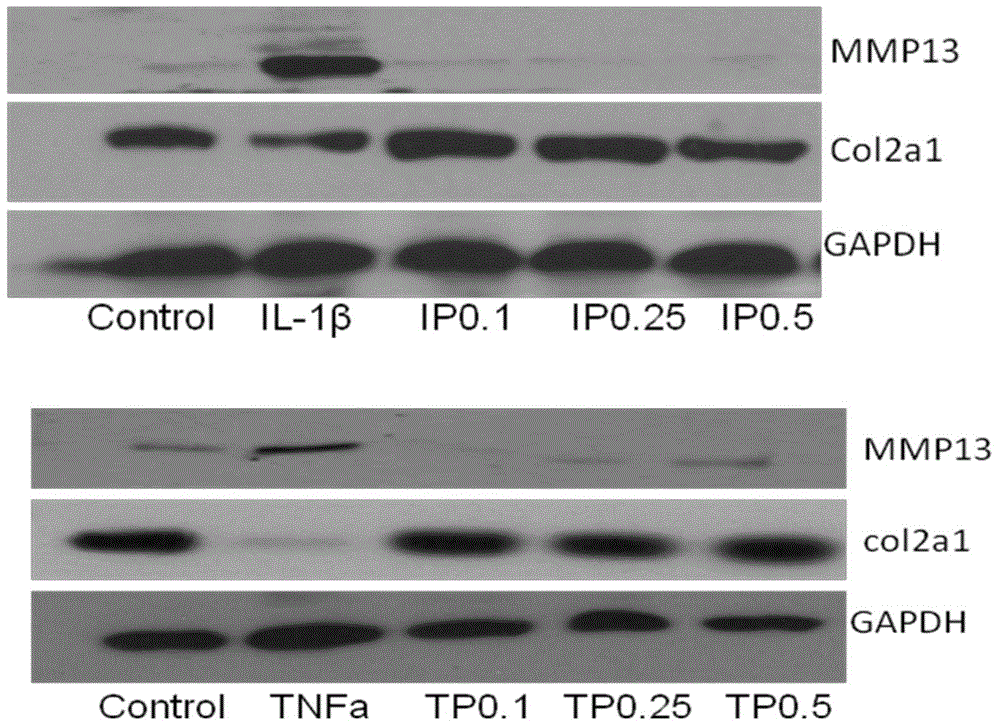
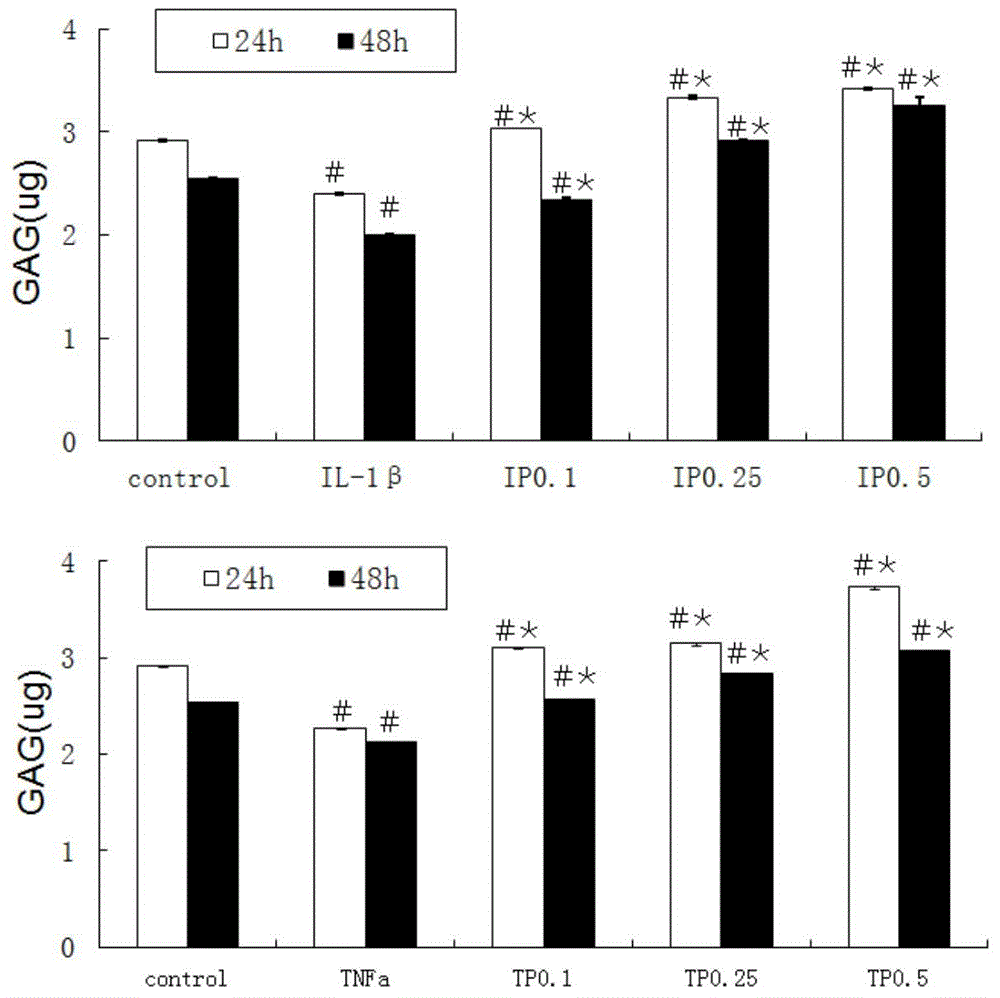
[0027] 2. The cells were divided into 5 groups: control group, IL-1β group (50ng / ml, peprotech company), IP0.1 group [IL-1β+PD0325901 (0.1μM)], IP0.25 group [IL-1β +PD0325901 (0.25μM)], IP0.5 group [IL-1β+PD0325901 (0.5μM)], TNFa group (50ng / ml, peprotech company), TP0.1 group [TNFa+PD0325901 (0.1μM)], TP0 .25 group [TNFa+PD0325901 (0.25 μM)], TP0.5 group [TNFa+PD0325901 (0.5 μM)]. Among them, PD0325901 was purchased from santacruz company. The above drug concentrations and cytokines were added respectively. After 24 hours, the total cell protein was extracted for quantitative protein detection (Western blot method) MMP13 (santacruz company, product number SC-30073), Col2a1 (santacruz company, product number SC-52658) and the content of glycosaminoglycans in the cells.
[0028] 3. Quantitative protein detec...
Embodiment 2
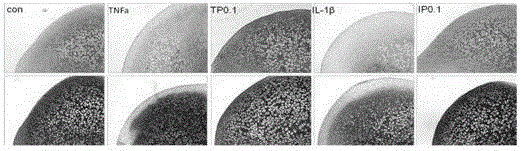
[0031]The femoral heads of SD rats at the age of 2 weeks were taken out (at this time, the femoral heads were not calcified and were all cartilage tissues). Divided into 5 groups: control group (con group), IL-1β group (50ng / ml, peprotech company), IP0.1 group [IL-1β+PD0325901 (0.1μM)], TNFa group (50ng / ml, peprotech company), TP0.1 group [TNFa+PD0325901 (0.1 μM)]. Among them, PD0325901 was purchased from santacruz company. The above drug concentrations and cytokines were added respectively. Change the medium every 48 hours until 96 hours, take out the femoral head, fix with 4% paraformaldehyde for more than 24 hours, carry out decalcification and dehydration, tissue embedding and paraffin sectioning.
[0032] In the femoral head cartilage tissue culture experiment in vitro, it was found that the IL-1β group (50ng / ml, peprotech company) and the TNFa group (50ng / ml, peprotech company) acted on inflammatory factors for 4 days, compared with the control group (con group ) Obvi...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com