Pyran as floral odorant
A dotted line, compound technology, applied in lily-of-the-valley-type fragrance ingredients, floral fragrance field, can solve the problems of no reported or implied sensory properties, no reported or implied compound application, etc.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
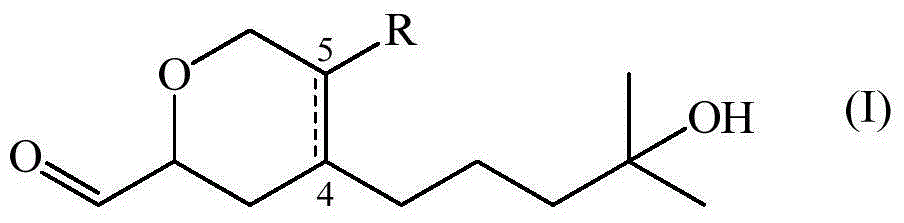
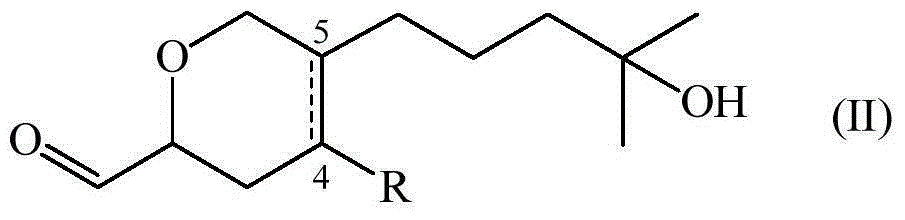
[0066] Synthesis of Compounds of Formula (I)
[0067] Contains 88% of 4-(4-hydroxy-4-methylpentyl)-3,6-dihydro-2H-pyran-2-carbaldehyde and 12% of 5-(4-hydroxy-4-methylpentyl)-3,6-dihydro-2H-pyran-2-carbaldehyde The composition of (also referred to as composition 1)
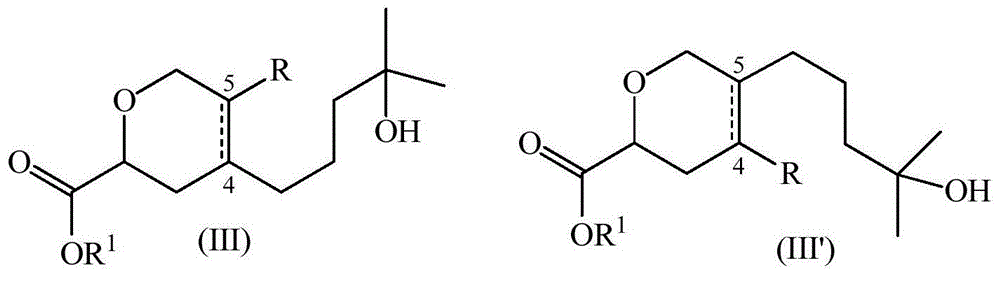
[0068] a) ethyl 4-(4-hydroxy-4-methylpentyl)-3,6-dihydro-2H-pyran-2-carboxylate (as main compound) and 5-(4-hydroxy-4- Methylpentyl)-3,6-dihydro-2H-pyran-2-carboxylic acid ethyl ester (as a minor compound)
[0069] A mixture of ethyl glyoxylate (50% in toluene) (38.6 mL, 194 mmol) and myrcenol (20 g, 130 mmol) was heated at reflux for 29 hours. Excess ethyl glyoxylate and toluene were evaporated and the residue was purified by column chromatography on silica gel using a gradient mixture of heptane and ethyl acetate (85:15 to 60:40) to give the pure ester (80% yield ), a colorless oil and an 88:12 mixture of regioisomers.
[0070] Major isomer:
[0071] 1 H NMR: 5.44(m,1H),4.36(m,1H),4.18-4.28(m,4H),2....
Embodiment 2
[0084] Preparation of Perfuming Compositions
[0085] A woody-citrus type cologne for men was prepared by mixing the following ingredients:
[0086]
[0087]
[0088] *in dipropylene glycol
[0089] 1) Pentadecenolactone; Source: Firmenich SA, Geneva, Switzerland
[0090] 2) Compounded perfume base; source: Firmenich SA, Geneva, Switzerland
[0091] 3) Methyl (1R)-cis-3-oxo-2-pentyl-1-cyclopentaneacetate; source: Firmenich SA, Geneva, Switzerland
[0092] 4) 5-(2,2,3-Trimethyl-3-cyclopentenyl)-3-methylpentan-2-ol; source: Givaudan SA, Vernier, Switzerland
[0093] 5) Propyl (S)-2-(1,1-dimethylpropoxy)propionate; source: Firmenich SA, Geneva, Switzerland
[0094] 6) Methyl cedryl ketone; source: International Flavors&Fragrances, USA
[0095] 7) (1S,2S,3S)-2,6,6-Trimethyl-bicyclo[3.1.1]heptane-3-spiro-2'-cyclohexen-4'-one; Source: Firmenich SA, Geneva,Switzerland
[0096] The addition of 400 parts by weight of Composition 1 (as described in Example 1) to the above...
Embodiment 3
[0098] Preparation of Perfuming Compositions
[0099] A floral, fruity, violet type perfuming composition was prepared by mixing the following ingredients:
[0100]
[0101]
[0102]
[0103] *in dipropylene glycol
[0104] **in isopropyl myristate
[0105] 1) 16-Hexadecanolide; Source: Firmenich SA, Geneva, Switzerland
[0106] 2) Pentadecalactone; Source: Firmenich SA, Geneva, Switzerland
[0107] 3) Tetrahydro-2-isobutyl-4-methyl-4(2H)-pyranol; source: Firmenich SA, Geneva, Switzerland
[0108] 4) 1,3,4,6,7,8-Hexahydro-4,6,6,7,8,8-hexamethyl-cyclopenta-g-2-benzopyran; source: International Flavors&Fragrances, USA
[0109] 5) Pentadecyl ester; source: Firmenich SA, Geneva, Switzerland
[0110] 6) cis-methyl dihydrojasmonate; source: Firmenich SA, Geneva, Switzerland
[0111] 7) (1S,1'R)-2-[1-(3',3'-dimethyl-1'-cyclohexyl)ethoxy]-2-methylpropyl propionate; source: Firmenich SA ,Geneva,Switzerland
[0112] 8) 3-(3,3 / 1,1-dimethyl-5-indanyl)propionaldehyde; s...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com