N-end-amino polyether-based acrylamide monomer and preparation method thereof
A technology of amino-terminated polyether-based acrylamide and double-terminated amino-based polyether, which is applied in the field of N-terminated amino-based polyether-based acrylamide monomer and its preparation, can solve the problem of disappearance of polymerizable parts, difficulty in popularization, and raw materials Problems such as high price, to achieve good hydration swelling, salt resistance, good inhibition ability, and wide application prospects
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
preparation example Construction
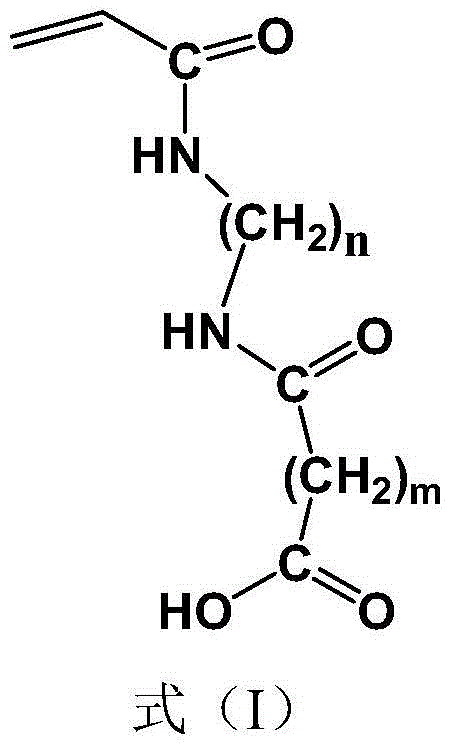
[0033] According to the preparation method provided by the present invention, preferably, the contact between the double-terminated amino polyether represented by the formula (2) and the acryloyl halide represented by the formula (3) is carried out in the presence of an organic solvent and / or an acid-binding agent Further preferably, the contact between the double-terminated amino polyether represented by the formula (2) and the acryloyl halide represented by the formula (3) is carried out in the presence of an organic solvent and an acid agent, on a molar basis, The ratio of the double-terminated amino polyether: organic solvent: acid binding agent is preferably 1:15-25:1.0-1.05, more preferably 1:18-22:1.0-1.02.
[0034] The organic solvent can be various organic solvents that can be used for the condensation reaction of amino groups and acyl halides, as long as they do not adversely affect the reaction. Preferably, the organic solvent is one or more of dichloromethane, dich...
Embodiment approach
[0036]According to a preferred embodiment of the present invention, the preparation method of the N-terminated amino polyether acrylamide monomer comprises the following steps:
[0037] (1) Add double-terminated amino polyether, organic solvent and acid-binding agent solution with a concentration of 20-50% by weight into a reaction bottle equipped with a cooling device, cool down to below 5°C, and then slowly add acryloyl halide, During the addition process, the temperature should not exceed 15°C. After the acryloyl halide is added, react at a temperature below 10-20°C for 0.5-4 hours;
[0038] (2) Leave the reactant obtained in step (1) to stand, separate the organic phase, wash several times with aqueous sodium chloride solution (preferably at a concentration of about 10% by weight), remove the water-soluble substances therein, and then wash it with anhydrous sulfuric acid Sodium is dried, and the organic solvent is evaporated to obtain an orange-red viscous liquid—the crude...
Embodiment 1
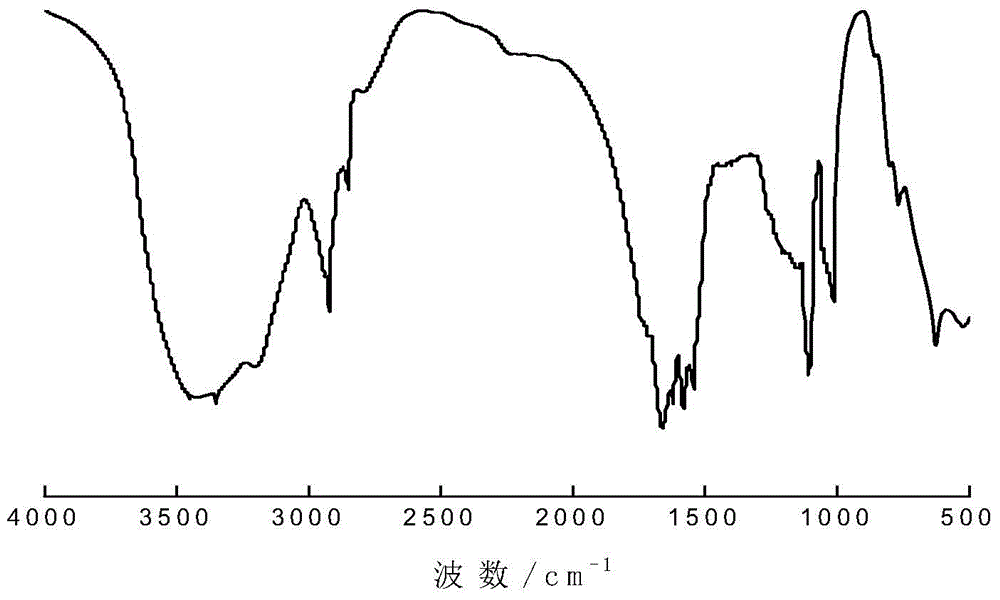
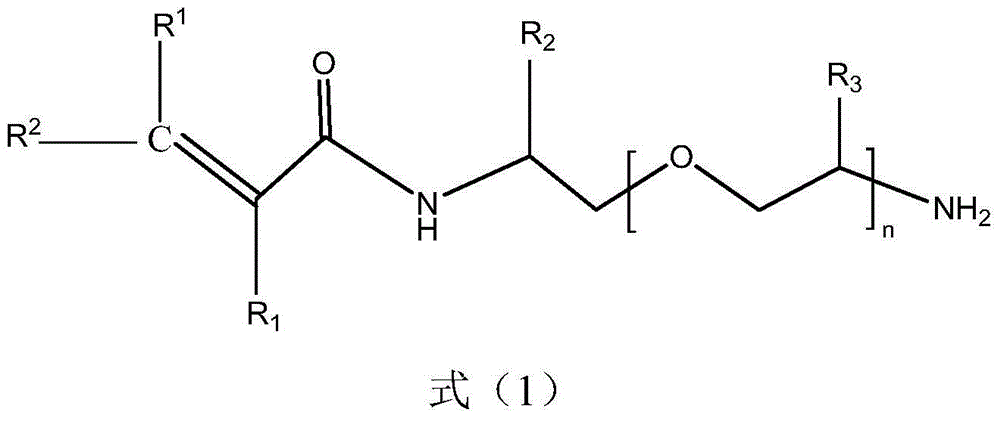
[0045] 1 mole of double-terminated amino polyether (structure as in formula (2), R 2 =R 3 =H, n=2), 15 moles of dichloromethane and 1 mole of sodium hydroxide (formed into an aqueous solution with a concentration of 40% by weight) were added to the reaction flask equipped with a cooling device, cooled to 5°C, and then slowly added 1 moles of acryloyl chloride, the temperature during the addition process does not exceed 15°C, after the addition of acryloyl chloride, react at a temperature below 15°C for 0.5h; let the obtained reactant stand still, separate the organic phase, and wash with a NaCl aqueous solution with a concentration of 10% by weight 3 times, dried with anhydrous sodium sulfate, evaporated the organic solvent to obtain an orange-red viscous liquid—the crude product, and washed the crude product with acetone to obtain a polymer grade product. The product was subjected to infrared spectroscopy. pass figure 1 It can be clearly seen that the primary amino peaks i...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com