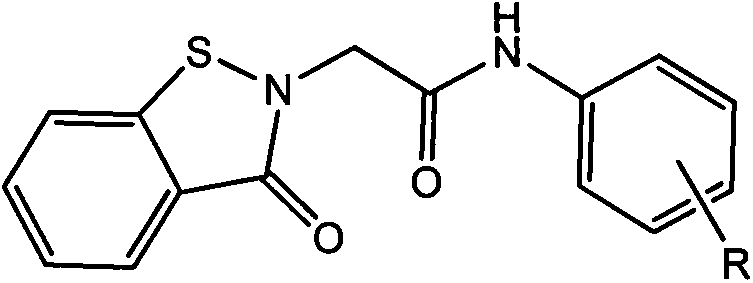
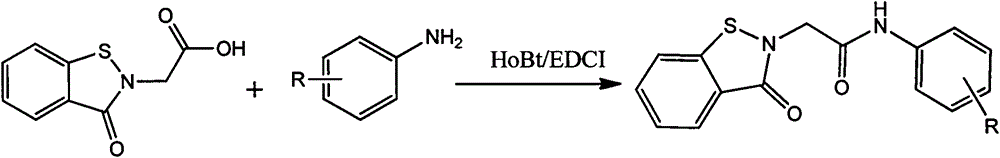Benzisothiazolone derivatives and synthetic method thereof
A technology of benzisothiazolinone and synthesis method, which is applied in the field of drug synthesis, can solve the problems of biological resistance and the like, and achieves the effects of good biological activity, simple reaction operation and high reaction yield
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
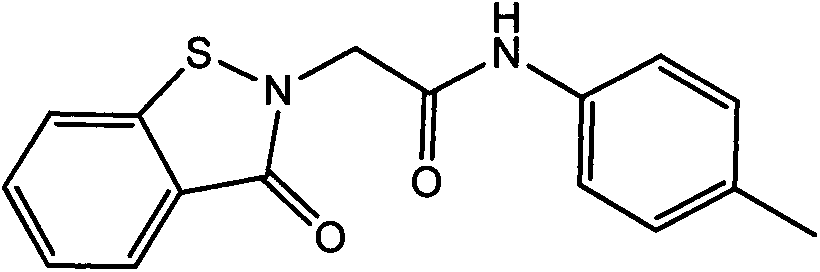
[0017] 4-Methylphenyl-1,2-benzisothiazolin-3-one-2-ylacetamide and its synthesis method
[0018] In a 250ml three-neck flask equipped with magnetic stirring, add 0.01mol of 4-methylaniline, 0.013mol of benzisothiazolinone acetic acid, add 0.012mol of HoBt and 0.012mol of EDCI as condensing agents, dissolve in DMF, and Reaction 20h. The reaction is complete. The solution was poured into water, and a large amount of solid precipitated out. Filtration, pickling, washing, drying, in crude products. Column chromatography gave 4-methylphenyl-1,2-benzisothiazolin-3-one-2-ylacetamide as a yellow solid, yield: 73%. Melting point: 159~160℃.
[0019]
[0020] 1 H NMR (DMSO-d6, 500MHz): 2.23 (S, 3H, CH 3 ), 5.11 (s, 2H, CH 2 ), 7.09-8.08 (m, 8H, H ring ), 10.11 (s, 1H, NH).
[0021] IR (cm -1 ): 3250, 3020, 1670, 1642, 1595, 1286, 1150, 648.
[0022] The bactericidal activity test shows that: when the compound concentration is 50ppm, the bacteriostatic rate of heterotrophic ...
Embodiment 2
[0024] 3-Chloro-1,2-benzisothiazolin-3-one-2-ylacetamide and its synthesis method
[0025] In a 250ml three-neck flask, add 0.01mol of 3-chloroaniline, 0.011mol of benzisothiazolinone acetic acid, and then add 0.011mol of HoBt and 0.013mol of EDCI as a condensation agent, dissolve completely with 15ml of DMSO, and react at room temperature for 16 hours. The reaction is complete. The solution was poured into water, and a large amount of solid precipitated out. Filtration, pickling, washing, drying, in crude products. Column chromatography gave 2-methoxyphenyl-1,2-benzisothiazolin-3-one-2-ylacetamide as a white solid, yield: 67%. Melting point: 176-177°C.
[0026]
[0027] 1 H NMR (DMSO-d6, 300MHz): 5.14(s, 2H, CH 2 ), 7.52-8.11 (m, 8H, H ring ), 10.28 (s, 1H, NH).
[0028] IR (cm -1 ): 3280, 3052, 1680, 1642, 1598, 1297, 1230, 1148, 648.
Embodiment 3
[0030] 2, 4 - Dimethylphenyl-1,2-benzisothiazolin-3-one-2-ylacetamide and its synthesis method
[0031] In a 250ml three-necked flask, 0.01mol of 2,4-dimethylaniline and 0.014mol of benzisothiazolinone acetic acid were added. Add 0.013 mol of HoBt and 0.011 mol of EDCI as a condensation agent, dissolve in DMSO, and react at room temperature for 24 hours. After the reaction was completed, the solution was poured into water, and a large amount of solids were precipitated. Filtration, pickling, washing, drying, in crude products. Column chromatography gave 2,4-dimethylphenyl-1,2-benzisothiazolin-3-one-2-ylacetamide as a yellow solid, yield: 72%. Melting point: 179-181°C.
[0032]
[0033] 1 H NMR (DMSO-d6, 300MHz): 3.31(s, 3H, CH 3 ), 5.14 (s, 2H, CH 2 ), 6.96-8.10 (m, 7H, H ring ), 9.54 (s, 1H, NH).
[0034] IR (cm -1 ): 3245, 3021, 1672, 1614, 1593, 1275, 1151, 648.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com