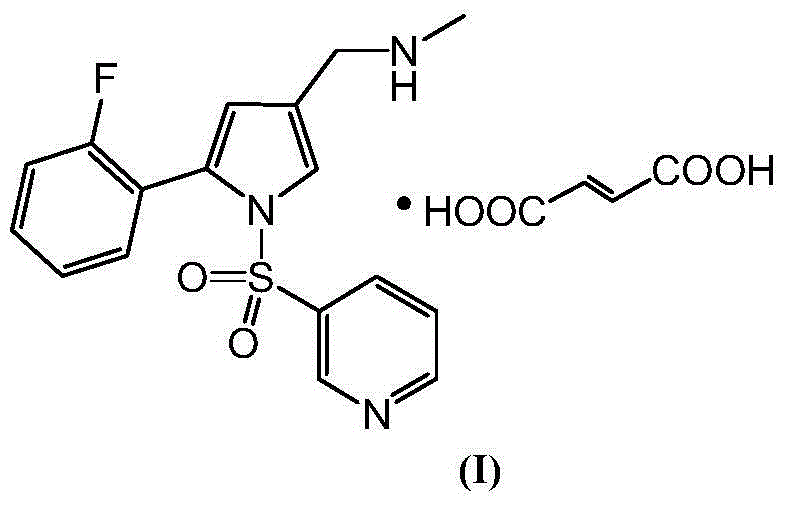
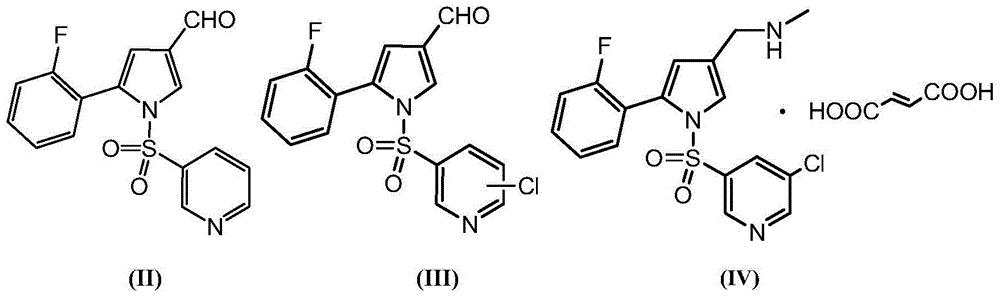
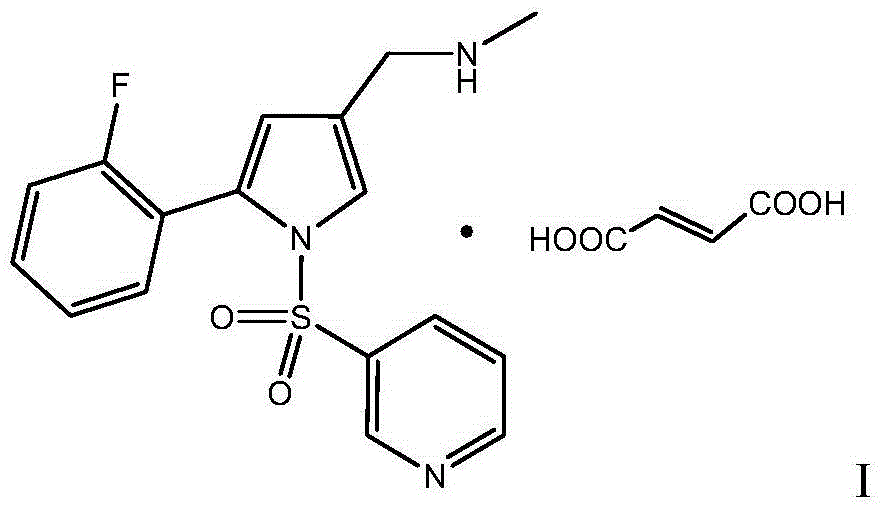
High-purity Vonoprazan Fumarate compound, intermediate and impurity thereof and preparation methods of high-purity Vonoprazan Fumarate compound, intermediate and impurity
A compound, high-purity technology, applied in organic chemistry and other fields
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0106] Preparation of 5-(2-fluorophenyl)-1-[(pyridin-3-yl)sulfonyl]-1H-pyrrole-3-carbaldehyde
[0107] At 20-25°C, the crude product (17g) was dissolved in dichloromethane (33mL), added dropwise with n-heptane (66mL), stirred for 30 minutes, filtered, and the filter cake was air-dried at 20-25°C for 3 hours to obtain Intermediate of Formula II 11.1 g. Yield: 65%, purity: 97.64%, impurity of formula III: 0.21%.
[0108] Preparation of Vonoprazan Fumarate (with reference to CN102421753A specification page 57 embodiment 5)
[0109] Add methanol (30.5mL), 5-(2-fluorophenyl)-1-[(pyridin-3-yl)sulfonyl]-1H-pyrrole-3- Formaldehyde (6.0 g, 18.3 mol), methanol solution of methylamine (2.5 g, 30-33% methylamine, 23.8 mol), stirred for 1.5 hours. Cool down to 0~10℃, add NaBH dropwise 4 (346mg, 9.16mol) of N,N-dimethylacetamide solution (9.1mL), the temperature in the system was controlled at 0-10°C during the dropwise addition, and the reaction was tracked by TLC after the dropwise ad...
Embodiment 2
[0113] Preparation of 5-(2-fluorophenyl)-1-[(pyridin-3-yl)sulfonyl]-1H-pyrrole-3-carbaldehyde
[0114] At 20-25°C, the crude product (17g) was dissolved in dichloromethane (33mL), added dropwise with n-heptane (99mL), stirred for 30 minutes, filtered, and the filter cake was air-dried at 20-25°C for 3 hours to obtain Product 11.9 g. Yield: 70%, purity: 97.57%, impurity of formula III: 0.25%.
[0115] Preparation of Vonoprazan Fumarate
[0116] Add methanol (30mL), 5-(2-fluorophenyl)-1-[(pyridin-3-yl)sulfonyl]-1H-pyrrole-3-carbaldehyde to a 200ml round bottom flask in sequence at 20-25°C (6.0 g, 18.3 mol), methanol solution of methylamine (2.1 g, 30-33% methylamine, 20.1 mol), stirred for 1.5 hours. Cool down to 0~10℃, add NaBH dropwise 4 (346mg, 9.16mol) of N,N-dimethylformamide solution (9.1mL), the temperature in the system was controlled at 0-10°C during the dropwise addition, and the reaction was tracked by TLC after the dropwise addition. 1N hydrochloric acid (35 mL)...
Embodiment 3
[0120] Preparation of 5-(2-fluorophenyl)-1-[(pyridin-3-yl)sulfonyl]-1H-pyrrole-3-carbaldehyde
[0121] At 20-25°C, the crude product (17g) was dissolved in dichloromethane (33mL), added dropwise with n-heptane (132mL), stirred for 30 minutes, filtered, and the filter cake was air-dried at 20-25°C for 3 hours to obtain Product 12.2g. Yield 72%, purity: 98.01%, chlorinated impurity of formula III: 0.22%.
[0122] Preparation of Vonoprazan Fumarate
[0123] Add methanol (30mL), 5-(2-fluorophenyl)-1-[(pyridin-3-yl)sulfonyl]-1H-pyrrole-3-carbaldehyde to a 200ml round bottom flask in sequence at 20-25°C (6.0g, 18.3mol), methanol solution of methylamine (2.5g, 30-33% methylamine, 23.8mol), stirred for 1.5 hours. Cool down to 0~10℃, add NaBH dropwise 4 (311mg, 8.24mol) of N,N-dimethylacetamide solution (9.1mL), the temperature in the system was controlled at 0-10°C during the dropwise addition, and the reaction was tracked by TLC after the dropwise addition. 1N hydrochloric acid ...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com