Exemestane intermediate and preparation method therefor and application thereof
A technology for exemestane and an intermediate, which is applied in the field of 6-aminomethyl-4-ene-3,17-androstanedione and its preparation, can solve the problem of low total yield, unsuitability for industrial production, Jones reagent is expensive and other problems, to achieve the effect of improving purity and yield
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0042] Example 1: Preparation of 6-(N-methyl-N-phenyl)-aminomethyl-4-ene-3,17-androstadione (1).
[0043] 20g of raw material androstenedione (2), 130ml of anhydrous tetrahydrofuran, 20ml of absolute ethanol, 20ml of triethyl orthoformate, 0.26g of p-toluenesulfonic acid in a four-necked bottle with a thermometer and mechanical stirring, at 40 The reaction was stirred at °C for 2 hours. 8 g of N-methylaniline and 2.2 g of paraformaldehyde were added, and the stirring reaction was continued for 7 hours. The solvent tetrahydrofuran was evaporated under reduced pressure below 40°C, 60 ml of methanol was added, stirred and dissolved at 40°C, cooled to 0°C, and stirred for 1 hour. After filtering and drying, a light yellow solid (1) was obtained. The crude product was 27.1 g, the liquid phase content was 95.5%, and the yield was 91%.
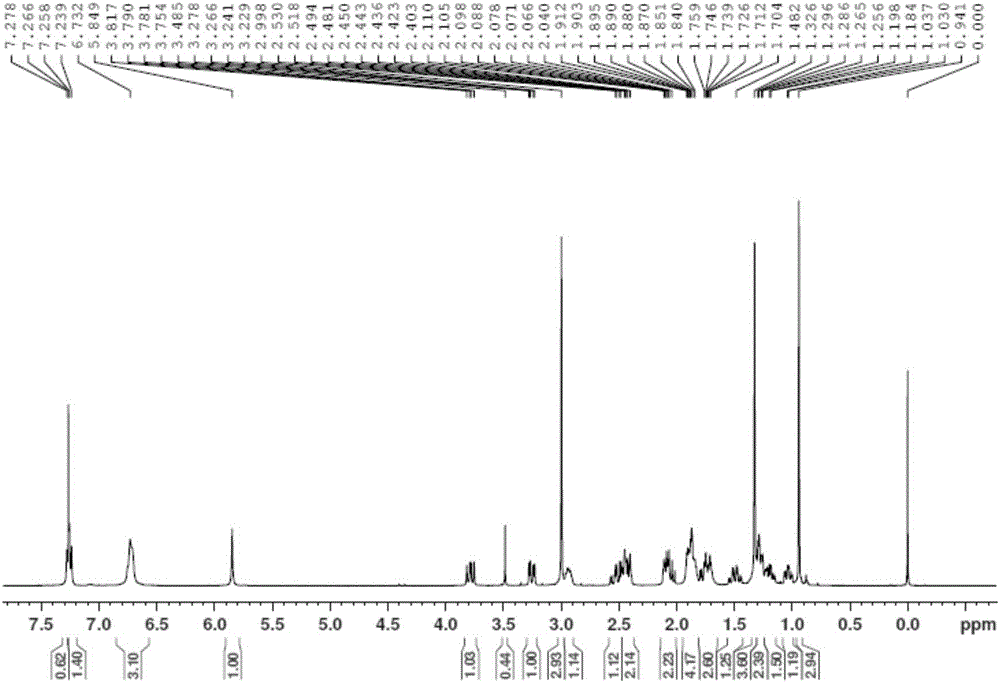
[0044] Product structure verification (see attached Figure 3-6 ):
[0045] 1 HNMR (δ, ppm, 400MHz, CDCl 3 ):7.239-7.278 (m, 2H, benzene ring); ...
Embodiment 2
[0049] Example 2: Preparation of 6-methylene-4-ene-3,17-androstadione (4) from (1).
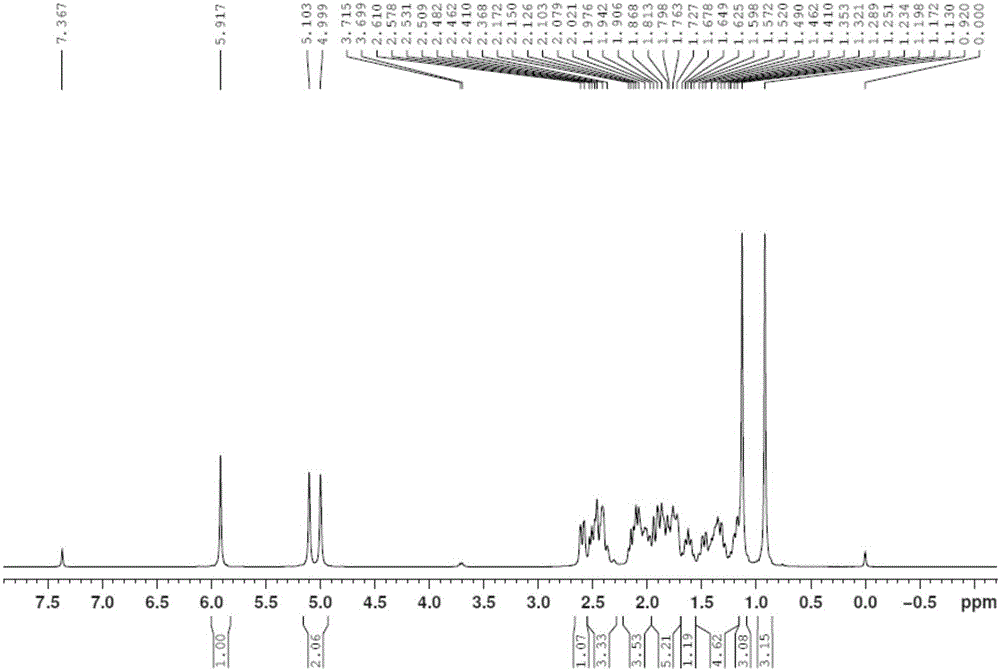
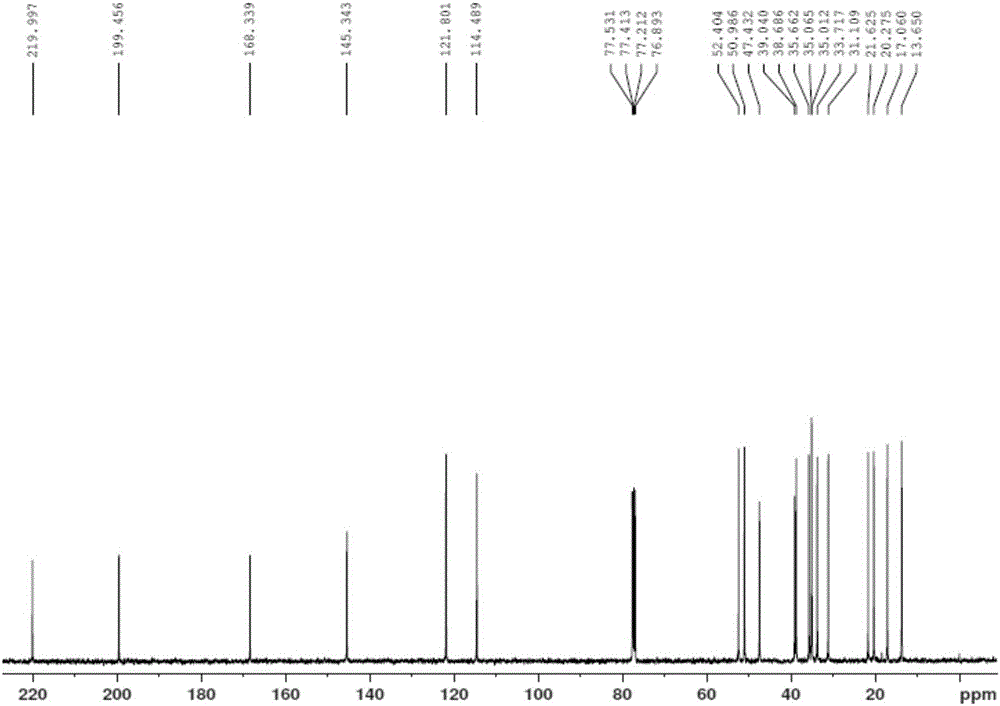
[0050] Mix 27 grams of the crude product 6-(N-methyl-N-phenyl)-aminomethyl-4-ene-3,17-androstadione (1) from the previous step, 36.5% hydrochloric acid and 90 milliliters of dichloromethane , stirred at 15-20°C to carry out the elimination reaction, the liquid phase followed the reaction process, and the reaction was completed in about 3 hours. The crude product of androstanedione (4) is 18.0 grams of light yellow powder, the liquid phase content is 92%, and the yield is 87%. Calculated from the raw material (2), the two-step total yield of the new process is 79.5%, and the product content is 92%. Product structure verification (see attached figure 1 ,2):
[0051] 1 HNMR (δ, ppm, 400MHz, CDCl 3 ):5.917(s,1H,CH=C); 5.103,4.999(s,2H,C=CH 2 );2.578-2.610(m,1H);2.462-2.482(m,1H);2.366-2.531(m,2H);1.727-2.172(m,8H);1.572-1.678(m,1H);1.130(s ,3H,CH 3 ); 1.172-1.520(m,4H); 0.920(s,3H,CH 3 )...
Embodiment 3-8
[0053] Example 3-8: Experiment on the preparation conditions of 6-(N-methyl-N-phenyl)-aminomethyl-4-ene-3,17-androstanedione (1).
[0054] The amount of feed, the order of addition, and the reaction conditions are all the same as in Example 1, the difference being that the amount of methanol and the crystallization temperature were changed during the aftertreatment. The results are shown in Table 1.
[0055] Table 1: Using different methanol consumption and crystallization temperature, the results are as follows:
[0056]
[0057]
PUM
| Property | Measurement | Unit |
|---|---|---|
| degree of polymerization | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com