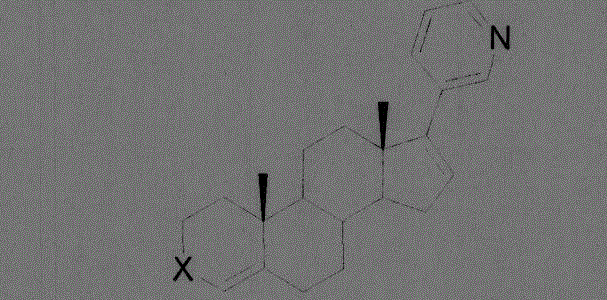
Compound used as CYP11B, CYP17 and/or CYP21 inhibitor
A CYP11B and compound technology, which is applied in the field of compounds as CYP11B, CYP17 and or CYP21 inhibitors, can solve the problems of drug treatment not being a treatment plan, drug deficiency, limitation of CYP11B1 activity and efficacy, etc.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
example 1
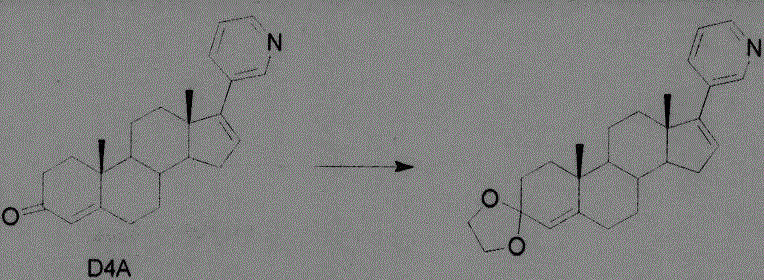
[0023] Example 1: Add 6.8 g of ethylene glycol to 34.7 g of D4A in 500 ml of anhydrous ether, cool down to -78 degrees, add 26.7 g of iodotriethylsilane dropwise, and react for 5 h. Cool down to room temperature, add 100ml of water to wash twice, dry over anhydrous sodium sulfate, evaporate the organic solvent under reduced pressure, and about 25g of the product crystallizes from dichloromethane.
[0024]
example 2
[0025] Example 2: Add 14.4 g of 2-mercaptoethylamine hydrochloride to 34.7 g of D4A in 500 ml of anhydrous methanol, and react at 35 degrees for 3.5 hours. Cool down to room temperature, add saturated sodium bicarbonate to raise the pH to about 8, add 100 ml of water to wash twice, dry over anhydrous sodium sulfate, evaporate the organic solvent under reduced pressure, and obtain about 32 g of the product crystallized from dichloromethane.
[0026]
example 3
[0027] Example 3: Add 8.5g of 2-mercaptoethanol to 34.7g of D4A in anhydrous 500ml of ether, fill it with hydrogen chloride gas at 20°C, and react for 0.5h. Saturated sodium bicarbonate was added to raise the pH to about 8, the organic phase was washed twice with 100 ml of water, dried over anhydrous sodium sulfate, the organic solvent was evaporated under reduced pressure, and about 35 g of the product was crystallized from dichloromethane.
[0028]
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D Engineer
- R&D Manager
- IP Professional
- Industry Leading Data Capabilities
- Powerful AI technology
- Patent DNA Extraction
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2024 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com