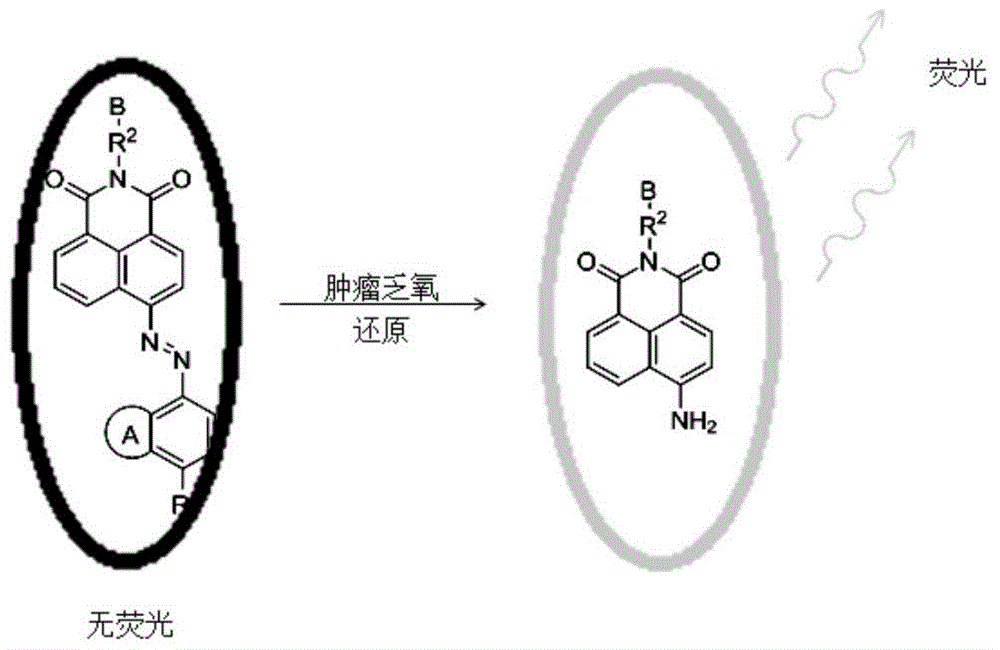
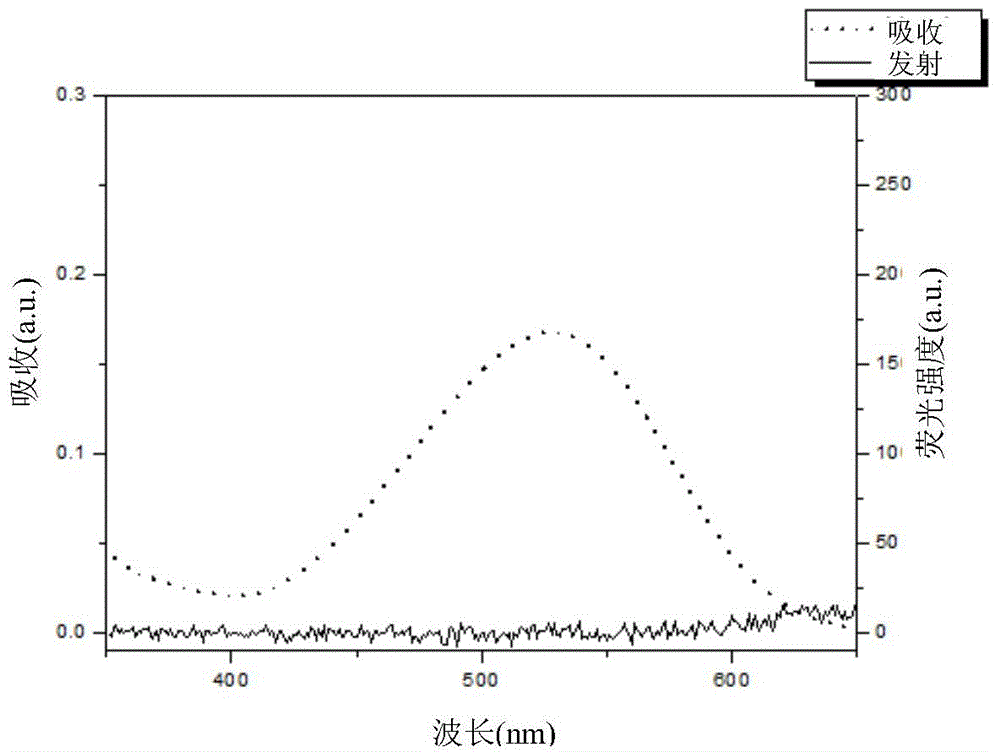
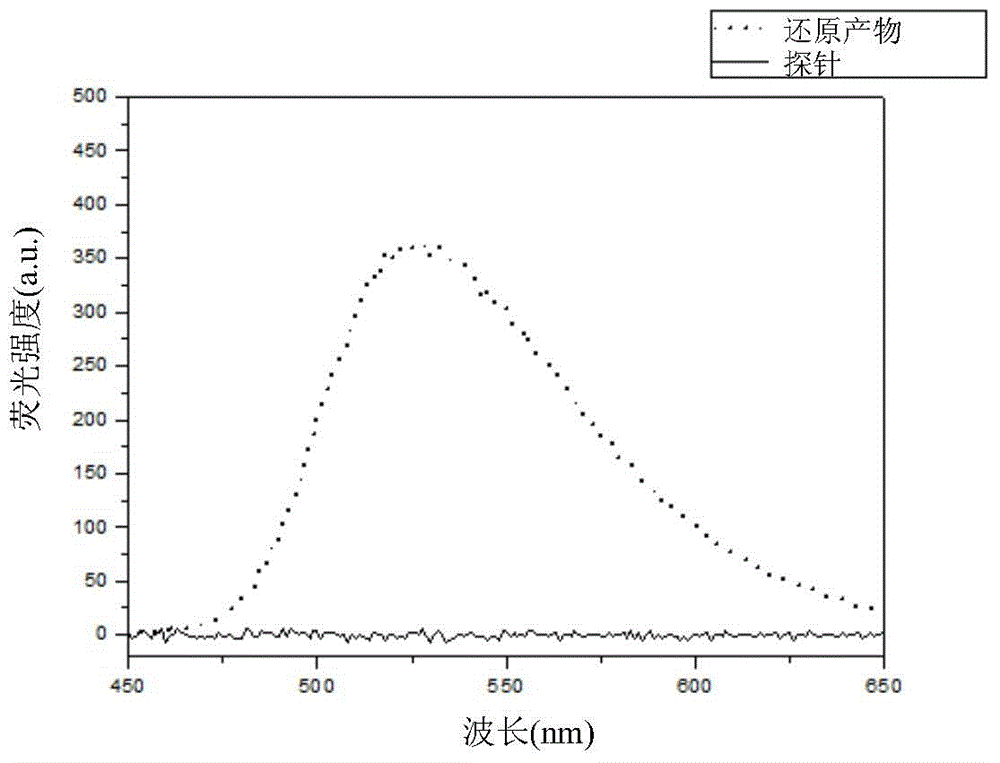
Compound for detecting tumor hypoxia and preparation method thereof
A compound and selected technology, applied in the field of fluorescent probes, can solve the problems of small changes in fluorescence and complex probe synthesis
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
preparation example Construction
[0068] The preparation method of fluorescent probe of the present invention comprises:
[0069] (1) In the presence of a solvent and a catalyst, 4-aminonaphthalene anhydride is prepared from 4-nitronaphthalene anhydride;
[0070] (2) In the presence of a solvent, from 4-aminonaphthalene anhydride and R 2 -NH 2 Compounds of the following formula II were prepared:
[0071]
[0072] (3) making the compound of formula II react in an ice bath in the presence of hydrochloric acid and sodium nitrite;
[0073] (4) Add the compound of the following formula III to the reaction solution of step (3) and react in an ice bath:
[0074]
[0075] (5) Remove the ice bath in step (4) to adjust the pH to about 6, and continue the reaction, so as to prepare the compound of formula I of the present invention that does not contain B.
[0076] To prepare a compound containing B, the method further comprises:
[0077] (6) In the presence of dichloromethane, triethylamine and HBTU (benzotri...
Embodiment 1
[0089] Embodiment 1: the synthesis of 4-aminonaphthalene anhydride
[0090]
[0091] Add 4-nitronaphthalene anhydride (980mg, 4mmol), 10mL absolute ethanol, stannous chloride (5.4g, 24mmol) and 6mL concentrated hydrochloric acid into a 25mL flask respectively, and heat to reflux for 5h under magnetic stirring. After the reaction, the heating was stopped, cooled naturally to room temperature, filtered with suction, washed with 5% dilute hydrochloric acid, washed with water, and dried by infrared to obtain 560 mg of a yellow solid with a yield of 65%. Solubility is too poor, no NMR. HRMS(ES-)calcd.for C 12 h 7 NO 3 [M-H]-212.0348,found212.0344.
Embodiment 2
[0092] Example 2: Synthesis of N-n-butyl-4-amino-1,8-naphthalimide
[0093]
[0094]Add 4-aminonaphthalene anhydride (430mg, 2mmol), 4mL DMF and n-butylamine (150mg, 2mmol) respectively into a 25mL flask, and heat to 110°C for 5h under magnetic stirring. Stop heating after the reaction is over, cool to room temperature naturally, pour the reaction solution into 100mL of potassium bisulfate solution (5%, m / m), filter with suction, wash with water, and dry by infrared. Separation by column chromatography (dichloromethane: methanol = 100:1) gave 390 mg of an orange solid, with a yield of 74%. 1 H NMR (CDCl 3 ,400MHz)δ8.59(d,1H,J=8.4Hz),8.39(d,1H,J=7.2Hz),8.17(d,1H,J=8.0Hz),7.62(dd,1H,J 1 =7.6Hz,J 2 =8.4Hz),7.40(s,2H),6.83(d,1H,J=8.4Hz),3.99(t,2H,J=7.6Hz),1.53–1.60(m,2H),1.27–1.36(m ,2H),0.90(t,3H,J=7.2Hz);HRMS(ES+)calcd.for C 16 h 16 N 2 o 2 [M+H] + 269.1290,found269.1292.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com