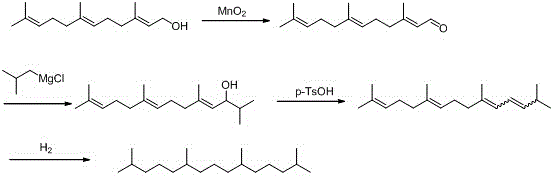
A kind of synthetic method of pristane
A synthesis method and pristane technology are applied in the field of biological reagents to achieve the effects of cheap raw materials, complicated operation and simple process
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
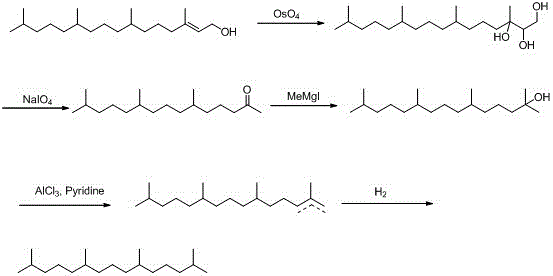
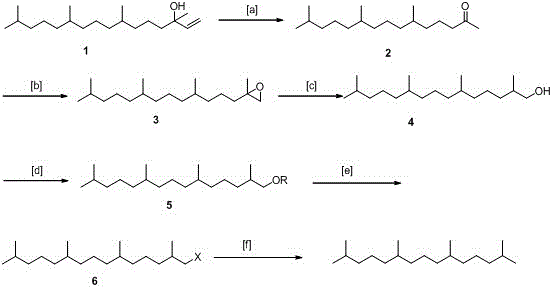
[0035] (1) Oxidation reaction, synthesis of phyton 2:
[0036] First, add 300g of isophytol 1, 3L of acetone, and 60ml of acetic acid into a 5L reaction flask in sequence. Slowly add 632g KMnO in batches 4 . With the prolongation of the reaction time, there will be exothermic phenomenon gradually. Cool with water circulation slightly until the addition is complete, continue the reaction for 5h, then heat at 60°C and continue the reaction for 5h, after the reaction is completed, filter, extract with ethyl acetate, and dry over anhydrous sodium sulfate , to obtain 262g phyton 2, yield: 96%, b.p.: 184°C / 11mmHg.
[0037] The NMR spectrum of Phytoketone 2 is:
[0038] 1 HNMR (500MHz, CDCl3 ): 2.43-2.39 (2H, t, CH2), 2.13 (3H, s, CH3), 1.54-1.50 (3H, m, CH), 1.26-1.25 (2H, m, CH2), 1.15-1.06 (16H, m, CH2), 0.89-0.84 (12H, m, CH3).
[0039] (2) Epoxidation reaction, synthesis of compound 3:
[0040] Add 190g of epoxidation reagent dimethyl methylene sulfonium sulfonium into a ...
Embodiment 2
[0054] (1) Oxidation reaction, synthesis of phyton 2:
[0055] First, add 300g of isophytol 1, 3L of acetone, and 60ml of acetic acid into a 5L reaction flask in sequence. Slowly add 545g H in batches 2 Cr 2 o 7 . With the prolongation of the reaction time, there is a gradual exothermic phenomenon, and the water is circulated and cooled slightly until the addition is completed, and the reaction is continued for 6 hours, and then heated at 50°C to continue the reaction for 8 hours, and the rest is the same as in Example 1, the yield: 97%.
[0056] (2) Epoxidation reaction, synthesis of compound 3:
[0057] Add 200g of dimethyl methylene sulfonium sulfonium into a 3L three-necked flask, fill it with N 2 , add 220ml of anhydrous DMSO, stir to dissolve, add 200g of phyton 2 and 1.5L of anhydrous THF in turn, stir for 30min, cool in an ice-water bath, add 48g of NaOH in batches, the color of the reaction system changes: brown-green-yellow, the addition is complete , The ice b...
Embodiment 3
[0067] (1) Oxidation reaction, synthesis of phyton 2:
[0068] First, add 300g of isophytol 1, 3L of acetone, and 60ml of acetic acid into a 5L reaction flask in sequence. Slowly add 450g NaIO in batches 4 . With the prolongation of the reaction time, there is a gradual exothermic phenomenon, and the water is circulated and cooled slightly until the addition is completed, and the reaction is continued for 4 hours, and then heated at 80° C. for 4 hours. The rest is the same as in Example 1, and the yield is 93%.
[0069] (2) Epoxidation reaction, synthesis of compound 3:
[0070] 180g dimethylsulfide ylide was added to a 3L three-neck flask, filled with N 2 , add 180ml of anhydrous DMSO, stir to dissolve, add 180g of phyton 2 and 1.5L of anhydrous THF in turn, stir for 30min, cool in an ice-water bath, add 32gNaH in batches, the color of the reaction system changes: brown-green-yellow, the addition is complete, The ice bath was removed, the temperature was slowly raised to ...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com