Crystal form of N-(4-fluorobenzyl)-N-(1-methyl piperidine-4-yl)-N'-(4-(2-methylpropanolato)-phenylmethyl)urea hemitartrate and preparation method thereof
A technology of methyl propoxy and hemi-tartrate, which is applied in the field of chemical medicine to achieve the effect of improving bioavailability, low humidity and good repeatability
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0069] Preparation of Form I:
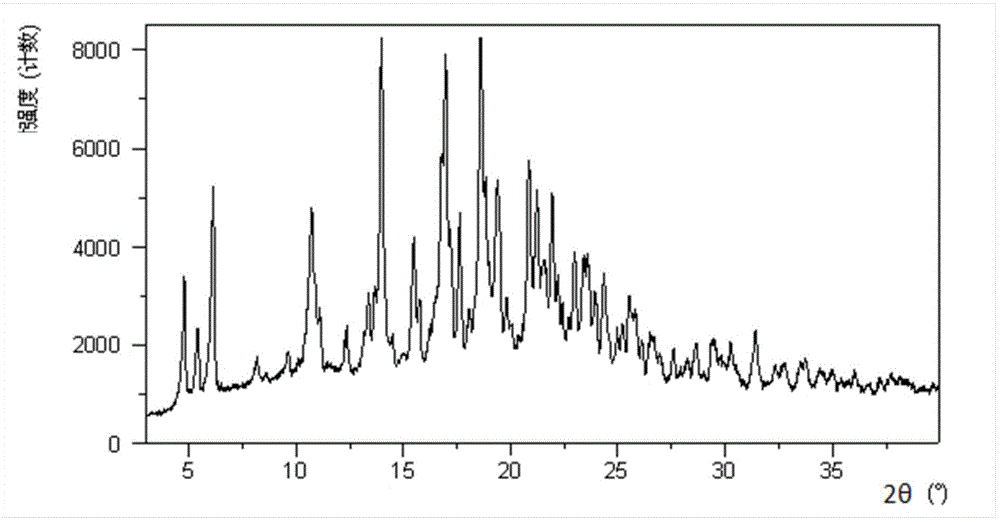
[0070] 504.3 mg of N-(4-fluorobenzyl)-N-(1-methylpiperidin-4-yl)-N'-(4-(2-methylpropoxy)-phenylmethyl) The powder of urea hemitartrate was dissolved in 10mL of water, placed in the refrigerator to freeze overnight, freeze-dried for 6 hours, and the solid obtained by freeze-drying was added to 16mL of dichloromethane / n-heptane mixed solvent with a volume ratio of 1:4, at room temperature The crystal form I can be obtained by stirring under the same conditions for 6 days. Table 1 shows the X-ray powder diffraction data of Form I obtained in this example.
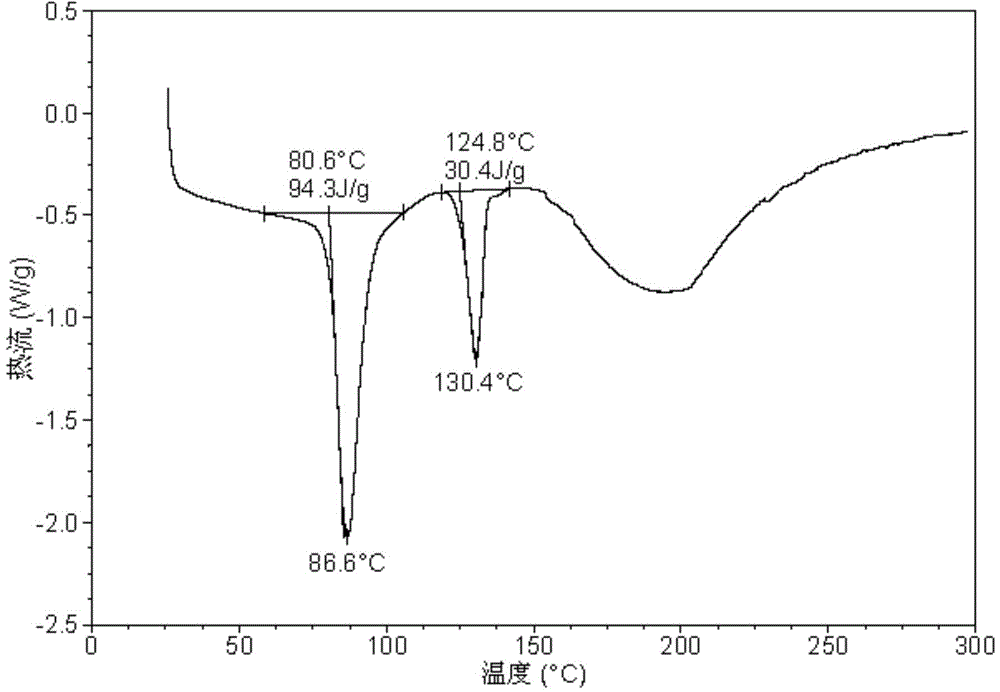
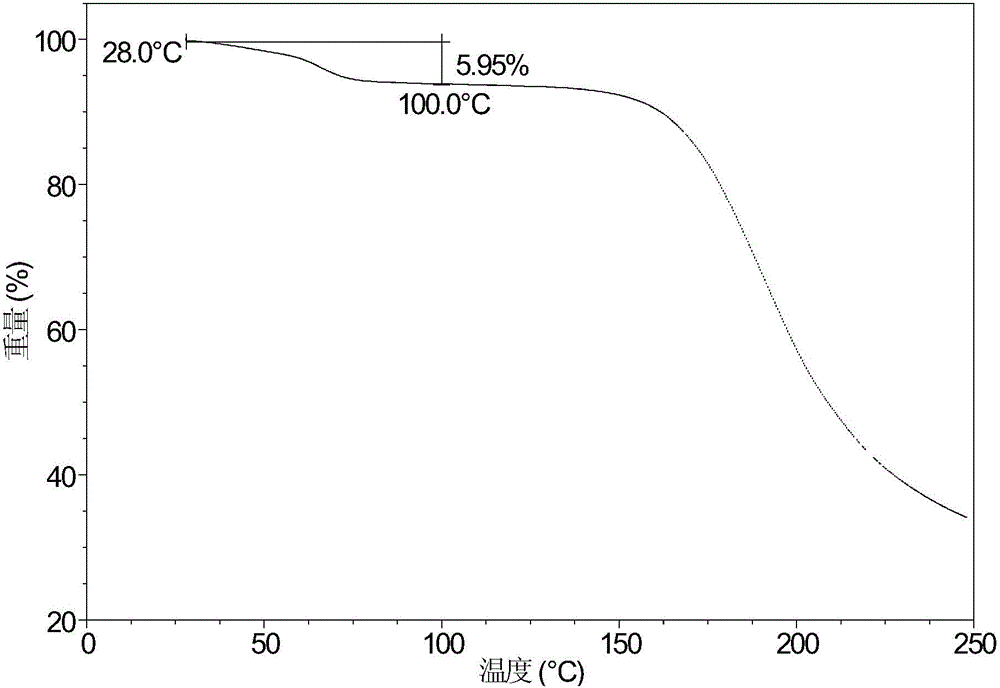
[0071] Its X-ray powder diffraction pattern is as figure 1 As shown, its differential scanning calorimetry diagram is shown in figure 2 As shown, its thermogravimetric analysis diagram is shown in image 3 .
[0072] Table 1 X-ray powder diffraction data of crystal form Ⅰ
[0073] 2theta d interval Relative Strength% 4.87 18.15 36.81 5.49 16.10 22.82 6.22 14...
Embodiment 2
[0076] Preparation of Form I:
[0077] 1000.4 mg of N-(4-fluorobenzyl)-N-(1-methylpiperidin-4-yl)-N'-(4-(2-methylpropoxy)-phenylmethyl) Dissolve the powder of urea hemitartrate in 15 mL of water, freeze overnight in the refrigerator, and freeze-dry for 7 hours to obtain N-(4-fluorobenzyl)-N-(1-methylpiperidin-4-yl)-N' - (4-(2-Methylpropoxy)-phenylmethyl)urea hemitartrate amorphous powder. Then 22.56 mg of the amorphous solid was weighed and added to 1 mL of dichloromethane / n-heptane mixed solvent with a volume ratio of 1:4, and stirred at room temperature for 1 day to obtain. The X-ray powder diffraction data of the crystal form I obtained in this embodiment are shown in Table 2.
[0078] Table 2 X-ray powder diffraction data of crystal form Ⅰ
[0079] 2theta d interval Relative Strength% 4.76 18.56 100.00 5.39 16.38 49.24 6.12 14.43 74.55 10.76 8.23 17.93 13.99 6.33 32.21 15.49 5.72 16.88 16.98 5.22 33.60 17.65 5...
Embodiment 3
[0081] Stability study of crystal form Ⅰ:
[0082] Take two portions of N-(4-fluorobenzyl)-N-(1-methylpiperidin-4-yl)-N'-(4-(2-methylpropoxy)-phenylmethyl)urea The sample of hemitartrate crystal form I is exposed and placed in the constant temperature and humidity chamber of 25 ℃, 60% relative humidity and 40 ℃, 75% relative humidity respectively, after 60 days, sampling measures XRPD (X-ray powder diffraction) and HPLC ( Purity was measured by high performance liquid chromatography. The experimental results are shown in Table 3. Stability study XRPD comparison chart Figure 4 shown.
[0083] Table 3 Stability study of Form Ⅰ
[0084]
[0085]
[0086] The results showed that the crystalline form I was stored for two months under the conditions of 25°C, 60% relative humidity and 40°C, 75% relative humidity, the crystal form remained unchanged and the purity was very high. The test results show that the crystal form I of the present invention has good stability.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com