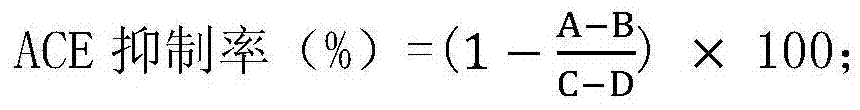Matsutake polypeptide, matsutake extract and their applications
A drug and a technology for preparing a drug, applied in the field of pine mushroom polypeptides, can solve problems such as side effects, taste dysfunction, etc., and achieve the effect of enriching the screening library and having great application value.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
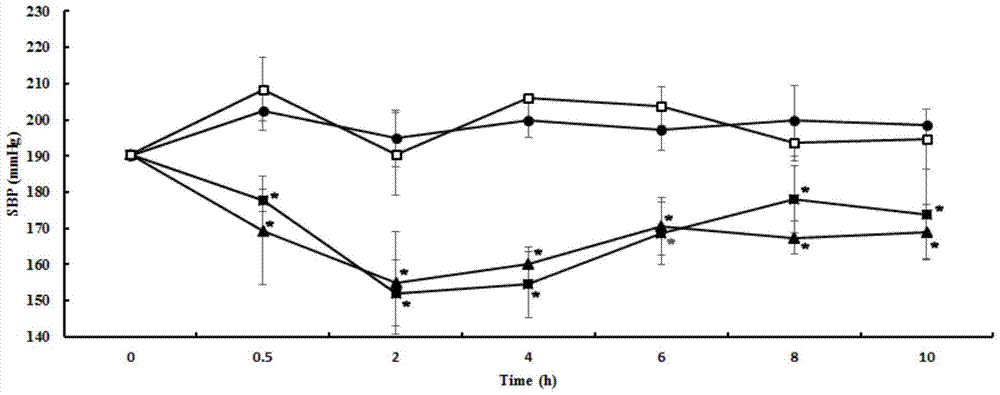
[0035] Embodiment 1, matsutake crude extract and its antihypertensive effect
[0036] 1. Preparation of matsutake crude extract
[0037] 1 part by mass of distilled water and 2 parts by mass of matsutake fruiting bodies were fully crushed into a homogenate, left standing at 4°C for 12 hours, then centrifuged at 8000 g for 15 minutes, and the supernatant was collected and freeze-dried to obtain a matsutake extract.
[0038] Every 14.52g matsutake fruiting body can get 400mg matsutake crude extract.
[0039] 2. Antihypertensive effect of matsutake extract on rats with essential hypertension (single-dose short-term experiment)
[0040] 1. Experimental animals
[0041] Primary hypertensive rats, male, 12 weeks old, body weight 240-270g. Animal testing facilities continue to maintain barrier environmental standards. The control range of the main environmental indicators: temperature 25±2℃, relative humidity 55±5%, light: dark=12h:12h. Animals were kept in standard boxes, with ...
Embodiment 2
[0051] Embodiment 2, discovery of matsutake polypeptide
[0052] 1. Determination of ACE inhibitory activity
[0053] 1. Preparation of ACE solution
[0054] Take 5 g of fresh rabbit lungs with connective tissue removed, wash them with pre-cooled normal saline, cut them into small pieces, and place them in 45 mL of pre-cooled sodium borate buffer containing 0.25 mM sucrose and 0.1 mM PMSF (pH 8.3, 0.1 M ), the homogenate was placed in sodium borate buffer (pH8.3, 0.1M) and dialyzed at 4°C for 12 hours, then centrifuged at 4°C and 9000g for 40 minutes, and the supernatant was collected, and the supernatant was 4°C and 9000g Centrifuge for 40 minutes, collect the supernatant (the supernatant is the ACE solution), and store it at -20°C for later use.
[0055] 2. Determination of ACE inhibitory activity (ACE reacts with HHL to generate hippuric acid, and the ACE inhibitory activity is estimated by the amount of hippuric acid produced)
[0056] 1. Mix 20 μL of the ACE solution p...
Embodiment 3
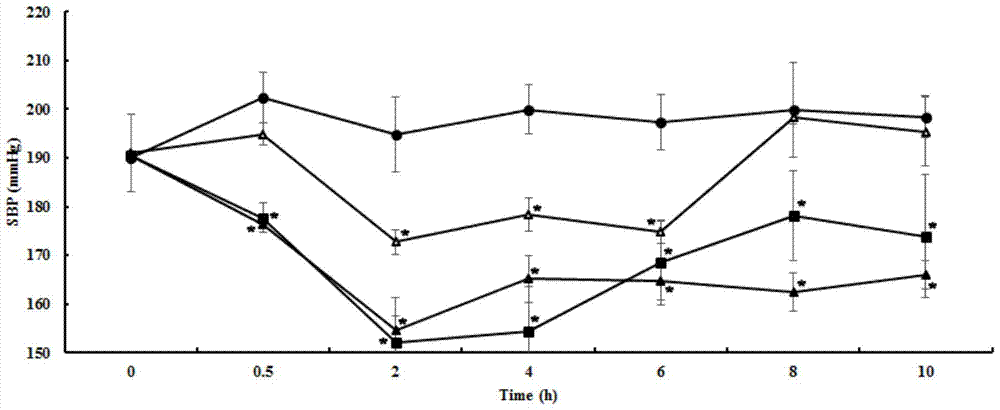
[0076] Example 3, the antihypertensive effect of matsutake polypeptide on essential hypertensive rats (single-dose short-term experiment)
[0077] The matsutake polypeptide shown in sequence 1 of the sequence listing was artificially synthesized (Shanghai Botai Biotechnology Co., Ltd.).
[0078] 1. Experimental animals
[0079] Primary hypertensive rats, male, 12 weeks old, body weight 240-270g. Animal testing facilities continue to maintain barrier environmental standards. The control range of the main environmental indicators: temperature 25±2℃, relative humidity 55±5%, light: dark=12h:12h. Animals were kept in standard boxes, with 2 rats per box. The sterilized bedding and cages were replaced twice a week, the environment inside the box was kept clean and dry, feed and drinking water were added daily, and animals were kept free to eat and drink. Quarantine and domestication process: The quarantine period for newly received animals is 3-6 days. During the quarantine per...
PUM
| Property | Measurement | Unit |
|---|---|---|
| molecular weight | aaaaa | aaaaa |
| weight | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com