Application of tetrahydroxanthone dimer compound to preparing alpha1-AR adrenergic receptor antagonist medicine
A technology of xanthone dimers, receptor antagonists, applied in the field of new uses of known compounds
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
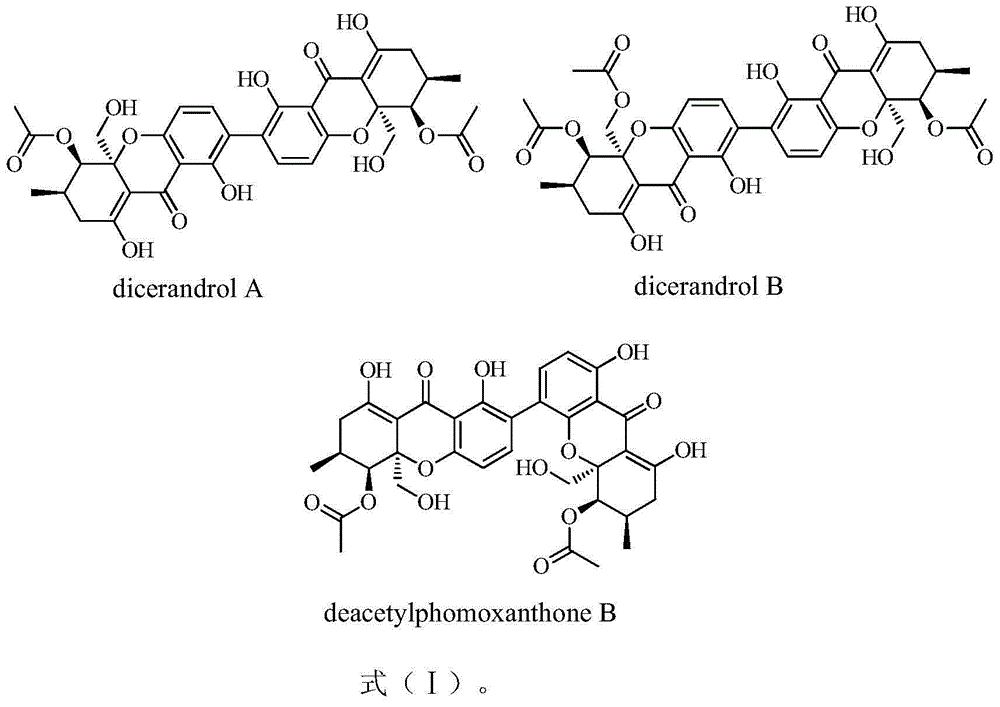
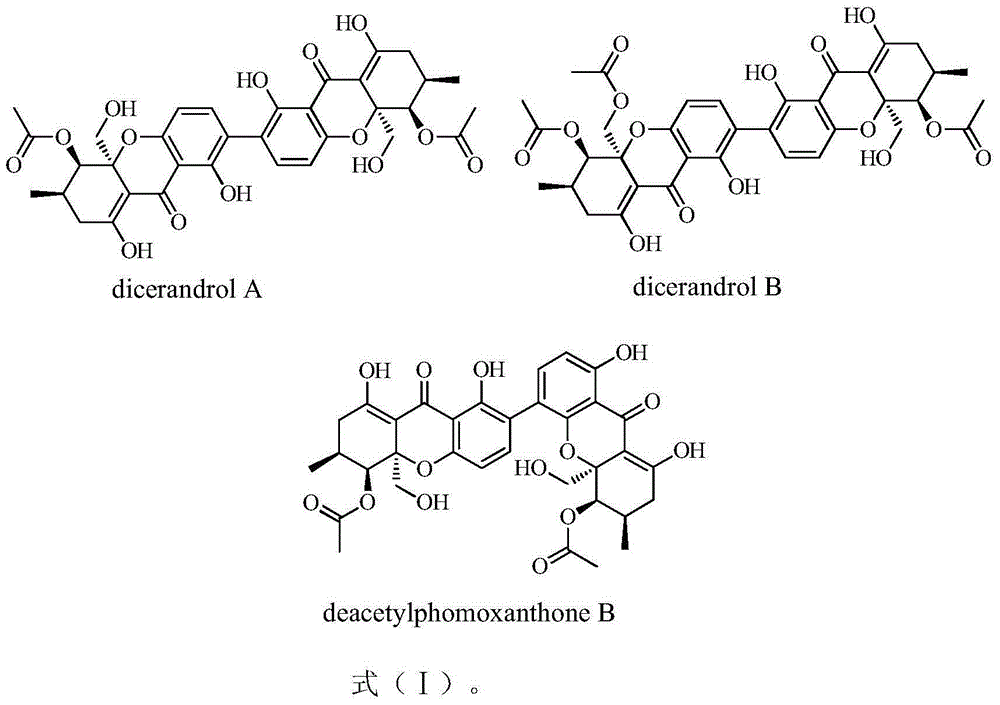
[0017] The tetrahydroxanthone dimer compound phomoxanthones of the present invention can be extracted and isolated from the fermentation and culture cells of the endophytic fungus Phomopsis sp.HNY29-2B of South China Sea mangrove, and its structure is shown in formula (I).
[0018]
[0019] The separation method of the above-mentioned tetrahydroxanthone dimer compound comprises the following steps:
[0020] S1. Seed culture of the fungus Phomopsis sp.HNY29-2B (the depository unit of the marine fungus Phomopsis sp.HNY29-2B is the China Center for Type Culture Collection CCTCC, the preservation number is CCTCC NO: M 2014395, and the preservation date is September 2014 On the 3rd, the preservation address is: China. Wuhan. Wuhan University; the classification is named Phomopsis HNY29-2B Phomopsis sp.HNY29-2B) The composition of the PDA medium is: glucose 2g, potato 20g (clarified liquid obtained from filter residue), crude Sea salt 0.3g, agar 1-1.5g, water 100mL; make a test t...
Embodiment 2
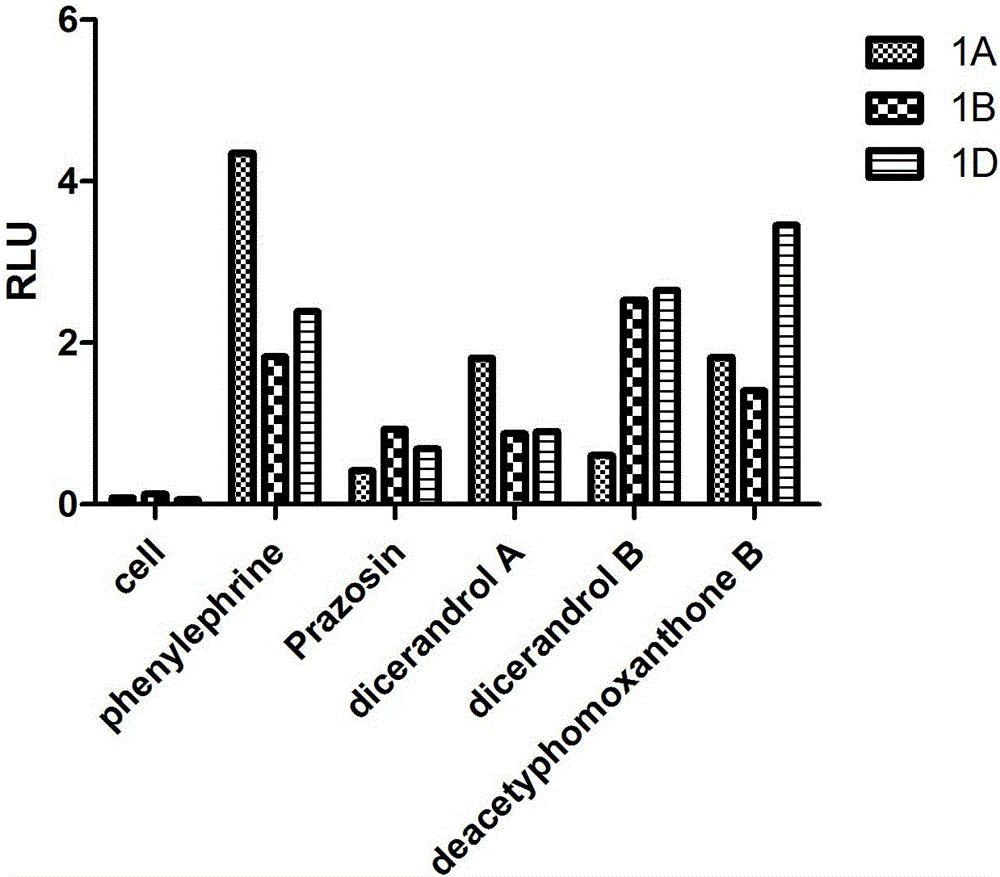
[0029] Tetrahydroxanthone dimer compounds dicerandrol A, dicerandrol B and deacetylphomoxanthone B on alpha 1 -Study on selective inhibition of AR subtypes:
[0030] 1. Strain cultivation and plasmid extraction: the corresponding plasmids (pGL4.29[luc2P / CRE / Hygro], pGL4.74[hRluc / TK], EX-A0967-M29, EX-Y3321-M29, EX-Y2008-M29 ) strains were added to LB liquid medium and cultured overnight; plasmids were extracted using a plasmid extraction kit (QIAprep Spin Miniprep Kit). And the plasmid concentration was measured with a miniature nucleic acid protein quantifier (Gene Company Limited, ND-100).
[0031] 2. Preparation of drug compound samples to be screened: Dissolve 1-2 mg of the pure compound in an appropriate amount of dimethyl sulfoxide to prepare a stock solution with a concentration of 2 mmol / L, take 0.5 uL to act on 99 uL containing transient cells In the culture medium, the final drug concentration was 10 μmol / L.
[0032] 3. Cell culture and plating: Take out HEK293 ce...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com