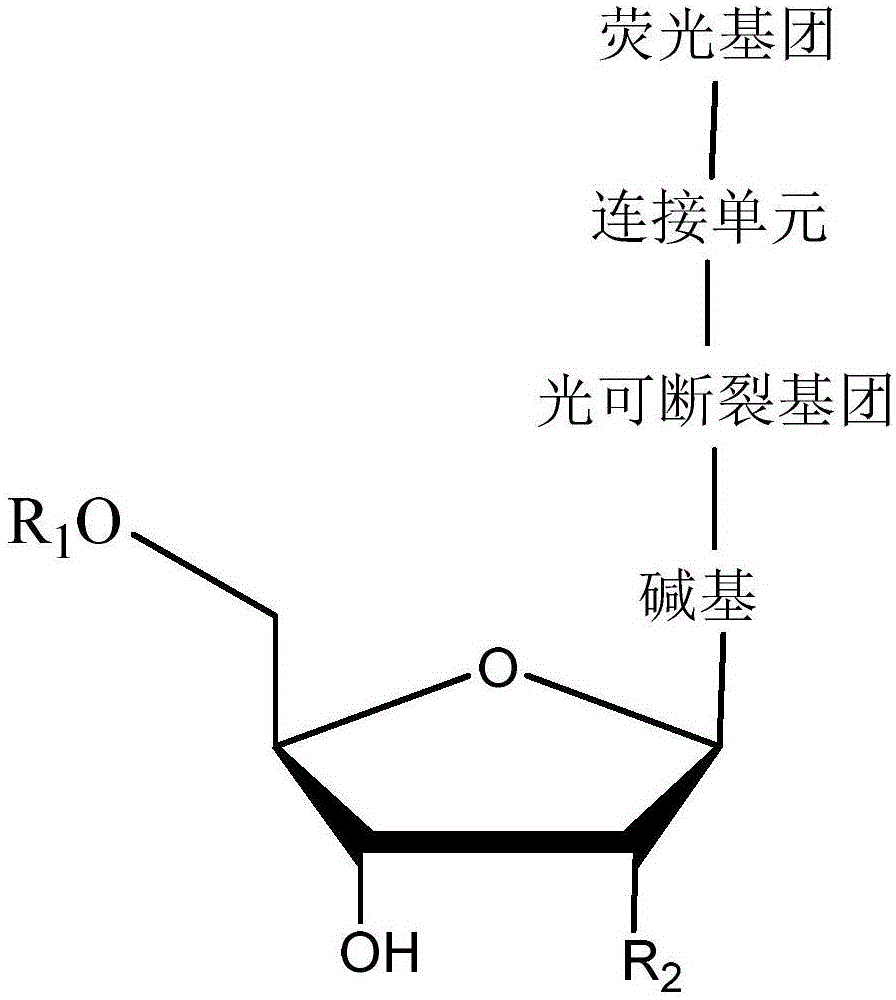
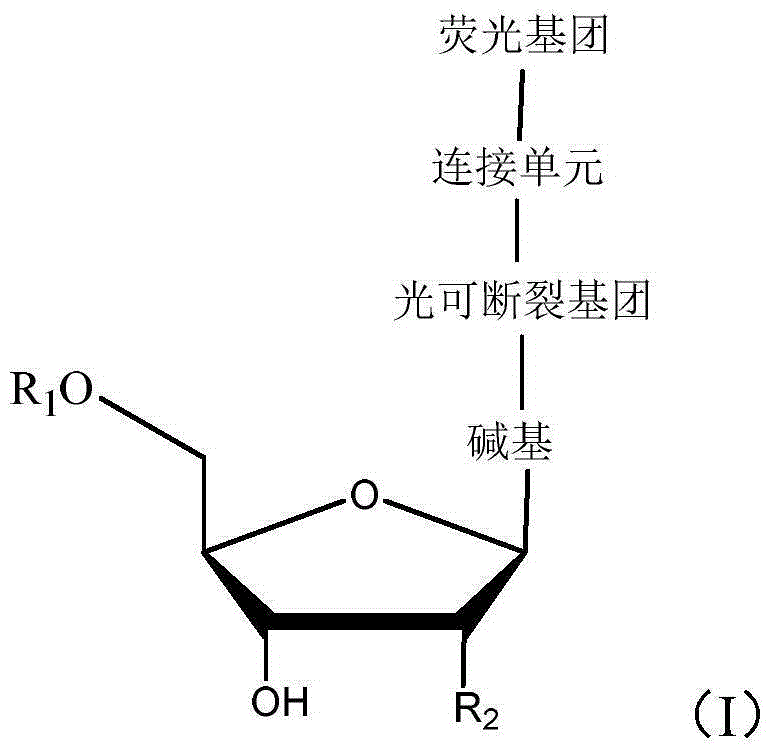
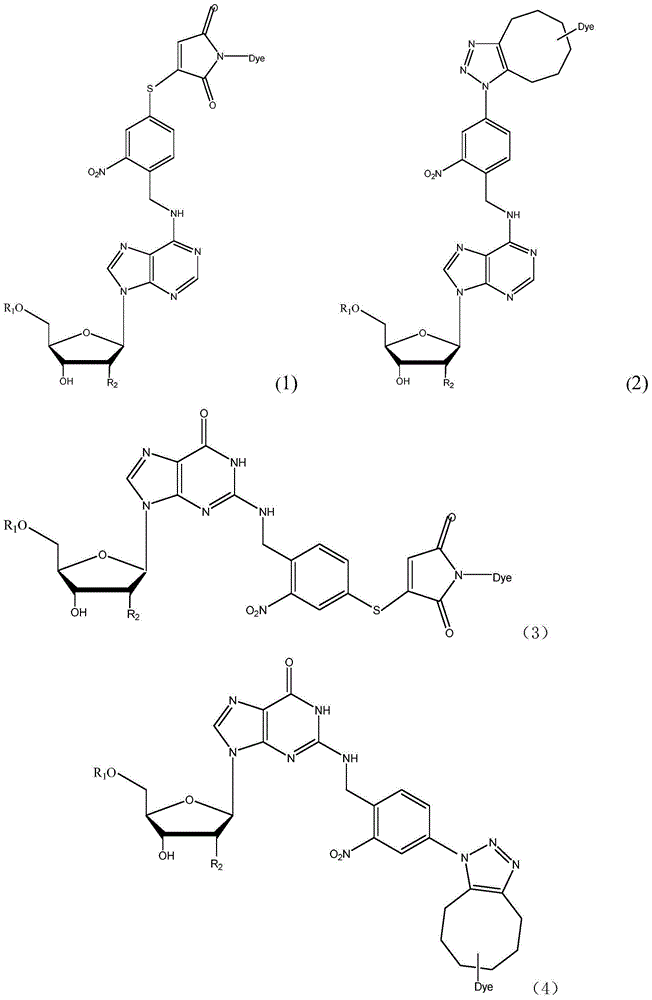
Light-fractured fluorescence-labeling reversible terminal compound and use thereof in DNA (Deoxyribonucleic Acid) or RNA (Ribonucleic Acid) sequencing
A fluorescent labeling and compound technology, applied in the field of medicine, can solve the problems of inability to identify DNA fragments, inability to attack phosphate groups, and reduce the length of sequencing, and achieve the effects of low price, increased yield, and reduced experimental difficulty.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0082] A method for synthesizing a photocleavable fluorescently labeled reversible terminal compound, comprising any one of the following routes 1-5:
[0083] Synthetic route 1:
[0084]
[0085] Wherein, the reaction conditions of step (i) are: compound A.1 in the presence of tert-butyldimethylsilyl chloride (TBSCl), imidazole (imidazole) and N,N-dimethylformamide (DMF), room temperature, overnight, and then the reaction solution was di-tert-butyl methyl dicarbonate ((Boc) 2 O), in the presence of 4-dimethylaminopyridine (DMAP) and N,N-dimethylformamide (DMF), at room temperature overnight, to obtain compound A.2;
[0086] The reaction condition of step (ii) is: compound A.2 is in Mg(ClO 4 ) 2 , and tetrahydrofuran THF, reacted under the conditions of existence to obtain compound A.3;
[0087] The reaction conditions of step (iii) are: compound A.3 exists in NaH, N,N-dimethylformamide (DMF) and 4-thio-2-nitrobenzyl bromide (4-sulfo-2-nitrobenzyl bromide) Compound A.4 ...
Embodiment 2
[0132] A method for synthesizing a photocleavable fluorescently labeled reversible terminal compound, comprising any one of the following routes 1-5:
[0133] Synthetic route 1:
[0134]
[0135] Wherein, the reaction condition of step (i) is: Compound A.3 is mixed with 4-iodo-2-nitrobenzyl bromide (4-iodo-2-nitrobenzyl bromide), NaH and N,N-dimethylformamide ( DMF) was reacted to obtain compound A.9 under the condition that exists;
[0136] The reaction condition of step (ii) is: compound A.9 is in NaN 3 , CuI, L-proline, NaOH and DMSO were reacted to obtain compound A.10;
[0137] The reaction condition of step (iii) is: compound A.10 is in SiO 2 Compound A.11 is obtained by reacting under the condition of existence;
[0138] The reaction condition of step (iv) is: compound A.11 is in n-tetrabutylammonium fluoride (n-Bu 4 NF), the reaction under the condition that THF exists obtains compound A.12;
[0139] The reaction condition of step (v) is: compound A.12 is in p...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com