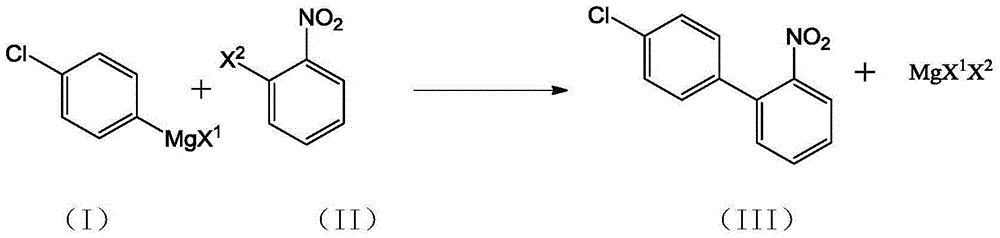
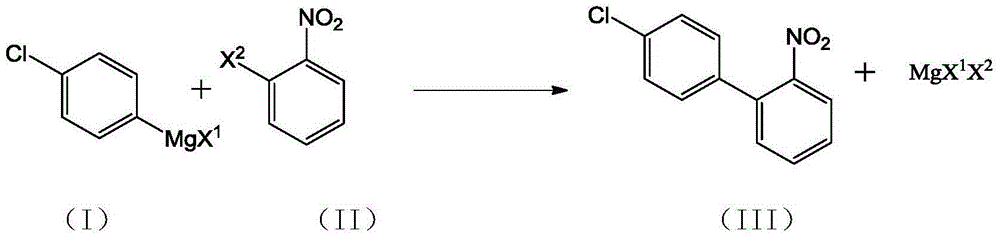
Preparation method of 4'-chloro-2-nitrobiphenyl
A technology of nitrobiphenyl and nitrohalobenzene is applied in the preparation of organic compounds, chemical instruments and methods, organic chemistry, etc., and can solve the problems of inflammability and explosion, low aromatization yield, complicated process steps, etc. problem, to achieve the effect of low production cost and short process steps
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0025] Dissolve 10.0g of 2-nitrochlorobenzene in 180mL of tetrahydrofuran, add 3.4g of 1,2-bis(diphenylphosphine)ethane nickel chloride, cool down to -45°C, and keep the system temperature at -40°C~-45°C Slowly add 98.7g of 11% tetrahydrofuran solution of p-chlorophenylmagnesium chloride in about 2 hours, keep it warm for 6 hours after dropping, remove the cold source, slowly rise to room temperature, add 30mL of toluene and 30mL of 20% hydrochloric acid, stir, and static layer Finally, the organic layer was evaporated to dryness to obtain a crude oil product, which was recrystallized using isopropanol to obtain 11.4 g of a yellow solid. After testing, the obtained yellow solid was 4'-chloro-2-nitrobiphenyl with a purity of 96.3%. The reaction yield is 74%.
Embodiment 2
[0027] Dissolve 10.0g of 2-nitroiodobenzene in 150mL of tetrahydrofuran, add 2.3g of 1,2-bis(diphenylphosphine)ethane nickel chloride, cool down to -78°C, and keep the system temperature at -78°C~-70°C Slowly add 45.7g of 15% tetrahydrofuran solution of p-chlorophenylmagnesium chloride in about 4 hours, keep it warm for 12 hours after dropping, remove the cold source, slowly rise to room temperature, add 30mL of toluene and 30mL of 20% hydrochloric acid, stir and statically separate Finally, the organic layer was evaporated to dryness to obtain a crude oil product, which was recrystallized using isopropanol to obtain 6.36 g of a yellow solid. After testing, the obtained yellow solid was 4'-chloro-2-nitrobiphenyl with a purity of 97.0%. The reaction yield is 66%.
Embodiment 3
[0029] Dissolve 10.0 g of 2-nitrochlorobenzene in 200 mL of tetrahydrofuran, add 6.8 g of 1,2-bis(diphenylphosphine)ethane nickel chloride, cool down to -50°C, and keep the system temperature at -45°C~-50°C Slowly add 108.5g of 10% tetrahydrofuran solution of p-chlorophenylmagnesium chloride in about 3 hours, keep it warm for 4 hours after dropping, remove the cold source, slowly rise to room temperature, add 30mL of toluene and 30mL of 20% hydrochloric acid, stir and statically separate Finally, the organic layer was evaporated to dryness to obtain a crude oil product, which was recrystallized using isopropanol to obtain 11.9 g of a light yellow solid. After testing, the obtained yellow solid was 4'-chloro-2-nitrobiphenyl with a purity of 97.5%. The reaction yield is 78%.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com