New method for preparing iopromide
A technology of iopromide and a new method, applied in the field of preparation of iopromide, can solve the problems of reducing compound polarity, low yield, large protecting group and the like
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
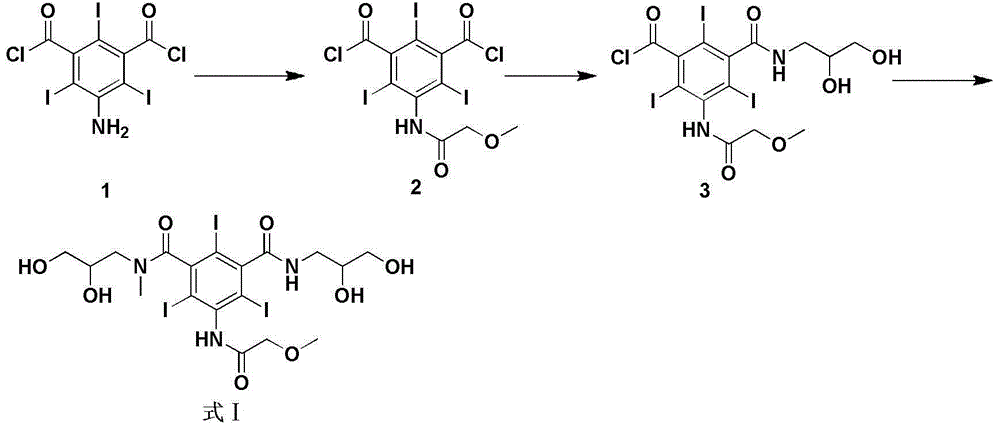
[0035] Embodiment 1: Synthesis of 5-methoxyacetamido-2,4,6-triiodoisophthaloyl chloride (formula III):
[0036] Dissolve methoxyacetyl chloride (54.6g, 0.50mol) in 100mL of dry DMA, add dropwise 5-amino-2,4,6-triiodoisophthalic acid dichloride (Formula II) at a temperature controlled at 10°C (100.0 g, 0.17 mol) of DMA solution in 300 mL, after dropping, react at room temperature for 24 hours. After the reaction is completed, the reaction solution is poured into ice water, and a white solid is precipitated immediately, stirred for 15 min, filtered with suction, and the filter cake is dissolved in dichloromethane, and then washed with saturated NaHCO 3 solution and saturated NaCl solution were washed twice each, and the organic layer was added with anhydrous NaCl 2 SO 4 After drying and desolvation, 101.1 g of white solid was obtained with a yield of 90.2%.
Embodiment 2
[0037] Embodiment 2: Synthesis of 5-methoxyacetamido-2,4,6-triiodoisophthalic acid (2,3-dihydroxypropyl)amide chloride (formula IV):
[0038] Dissolve 5-methoxyacetamido-2,4,6-triiodoisophthaloyl chloride (formula III) (60.0 g, 0.09 mol) in 400 ml DMA, add triethylamine (9.07 g, 0.09 mol) , the temperature was lowered to below -10°C, and 200ml of DMA-dissolved 3-amino-1,2-propanediol (8.18g, 0.09mol) solution was slowly added dropwise, and the reaction was continued at -10°C for 10h. Suction filtration, the filtrate was evaporated under reduced pressure to remove most of DMA, then CH was added dropwise 2 Cl 2 solution, a white solid was precipitated, filtered by suction, and dried to obtain 42.2 g of a white solid, with a yield of 65%.
Embodiment 3
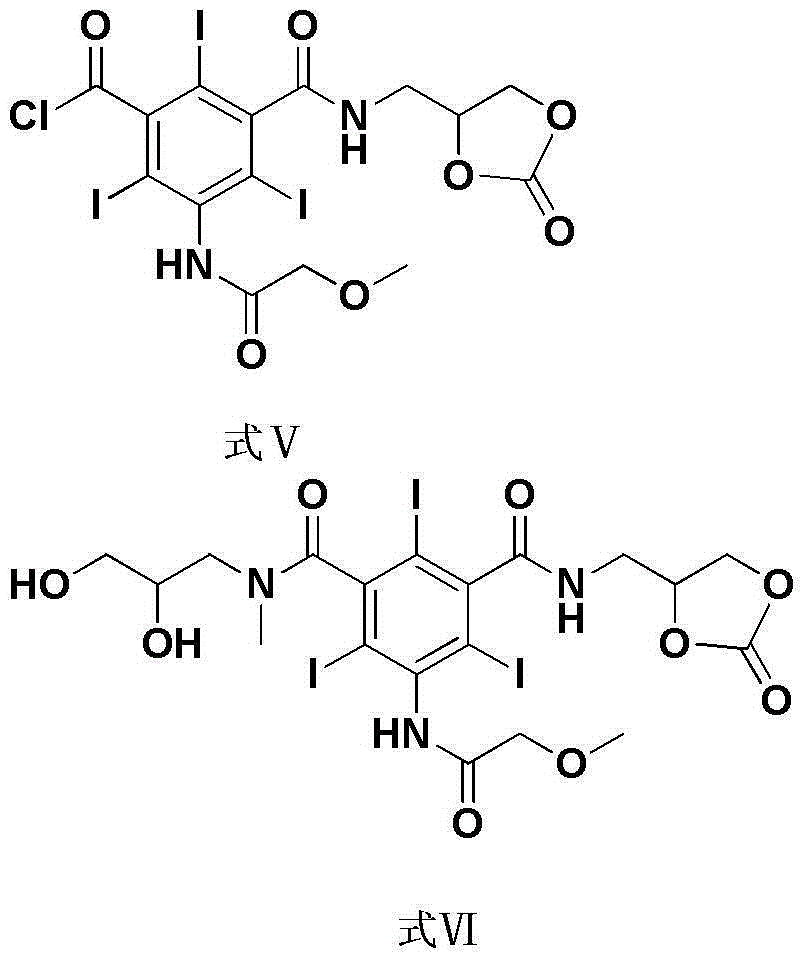
[0039] Example 3: 5-Methoxyacetamido-2,4,6-triiodoisophthalic acid [(2-oxo-1,3-dioxolan-4-yl)methyl]acyl
[0040] Synthesis of amine chloride (formula V):
[0041] 5-methoxyacetamido-2,4,6-triiodoisophthalic acid (2,3-dihydroxypropyl)amide chloride (formula IV) (40.0 g, 0.055 mol) was dissolved in 300 ml THF, After stirring and completely dissolving, solid phosgene (19.7 g, 0.066 mol) dissolved in 100 ml THF was added dropwise at room temperature. After the reaction was completed, evaporate to dryness under reduced pressure to obtain 39.4 g of off-white powder with a yield of 95%.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com