A method for reclaiming and utilizing bedaquiline stereochemical isomers
A technology of stereochemistry and isomers, applied in organic chemistry methods, chemical instruments and methods, organic chemistry, etc., can solve the problem that isomers cannot be reused
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
preparation example
[0049] Preparation of stereoisomers of 6-bromo-α-[2-(dimethylamino)ethyl]-2-methoxy-α-1-naphthyl-β-phenyl-3-quinoline ethanol
[0050] The preparation of step 1 intermediate N-(4-bromophenyl)-3-phenylpropanamide
[0051]
[0052] In a 500ml single-necked bottle, add p-bromoaniline (40g, 0.232mol) and dichloromethane (200ml), stir to dissolve, add triethylamine (32ml), add dropwise phenylpropionyl chloride (37.5g, 0.223mol) under ice cooling Stir the reaction overnight, pour the reaction solution into a large beaker, add an appropriate amount of 10% hydrochloric acid to precipitate a large amount of white flocculent solid, filter with suction, wash the filter cake with a small amount of ether, and dry to obtain a white solid N-(4-bromophenyl) -3-phenylpropanamide 62g, yield 92%. mp103-105°C.
[0053] Step 23- Preparation of Benzyl-6-bromo-2-chloroquinoline
[0054]
[0055] Add anhydrous DMF (32.6ml, 0.420mol) into a 500ml single-necked bottle, slowly add POCl dropwise...
Embodiment 1
[0069]
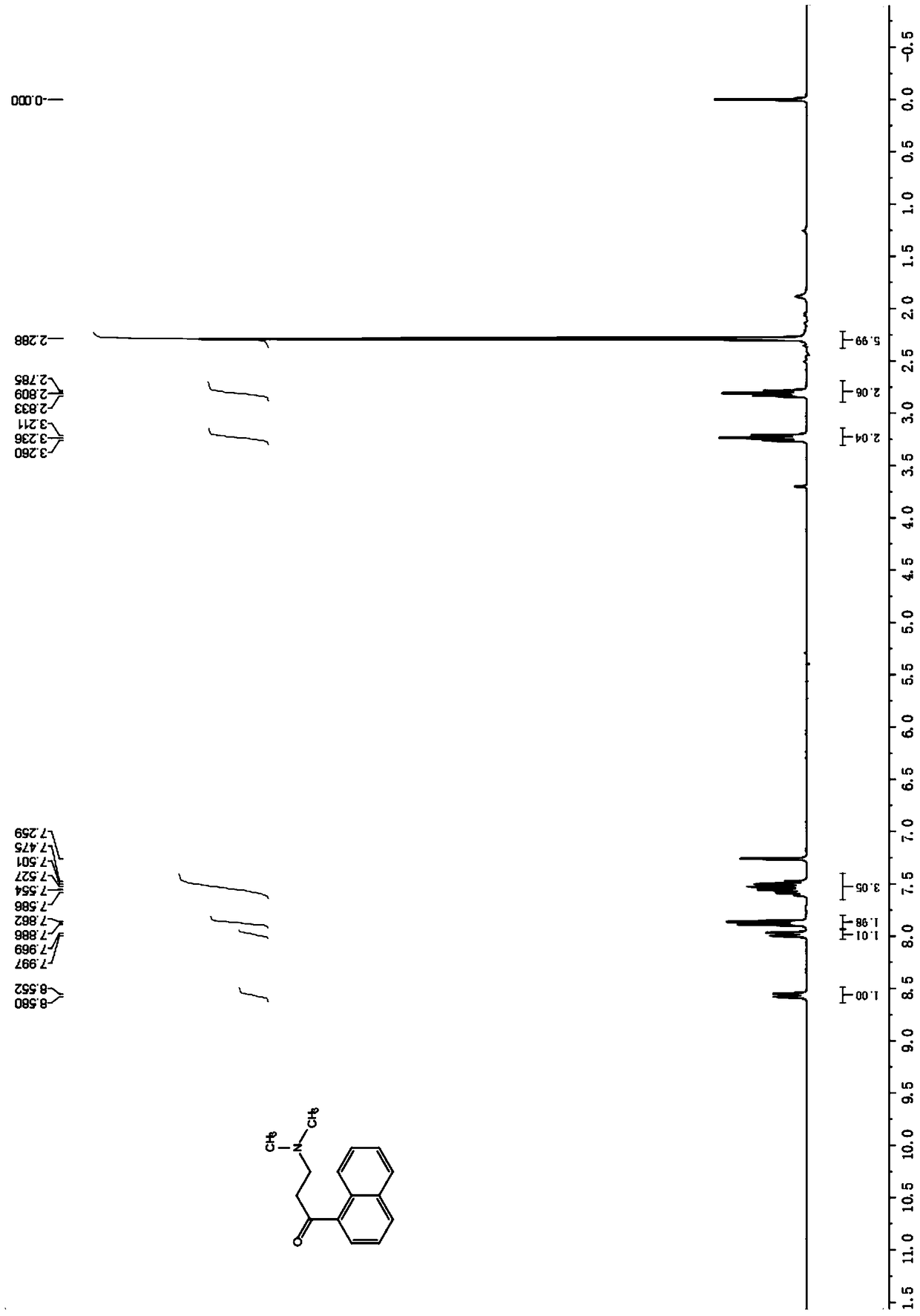
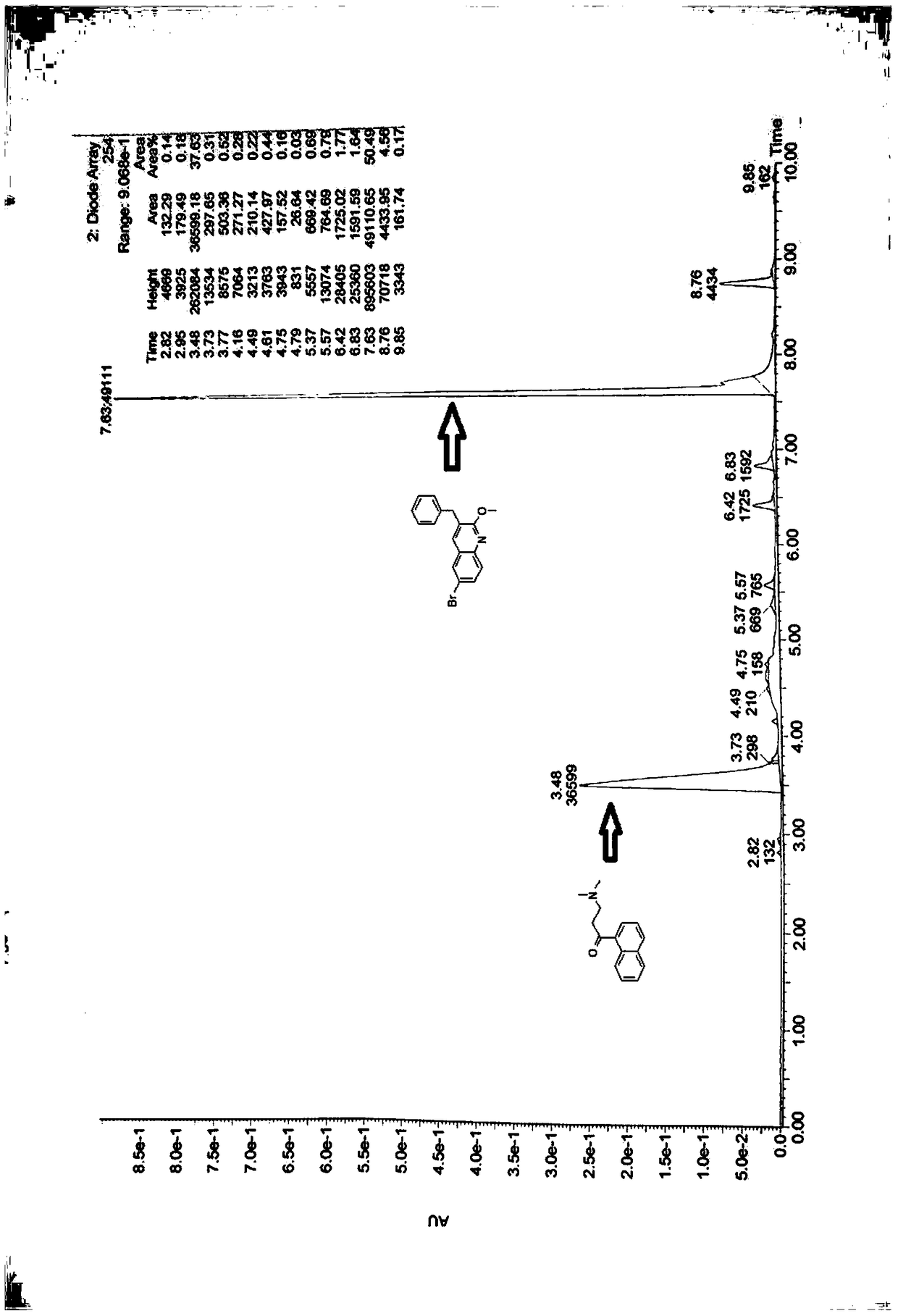
[0070] Diastereomer B (0.5g, 0.9mmol) was dissolved in 20ml of anhydrous THF, NaOH powder (90mg, 2.3mmol) was added at room temperature, reacted at room temperature for 2 hours, detected by TLC, after the reaction was complete (see attached image 3 ), adding 0.5M hydrochloric acid to adjust the pH to acidity, extracting with ethyl acetate, separating the organic phase and the aqueous phase, evaporating the organic layer to dryness under reduced pressure, and recrystallizing the residue with methanol to obtain 233 mg of 3-benzyl-6 bromo-2 - Methoxyquinoline (X), yield 80.5%. The aqueous phase was adjusted to alkaline with saturated sodium carbonate solution, extracted with ethyl acetate, the organic phase was separated, dried over anhydrous magnesium sulfate, and evaporated to dryness to obtain 168 mg of 3-dimethylamino-1-naphthyl-1-propanone (Y). Yield 82.3%.
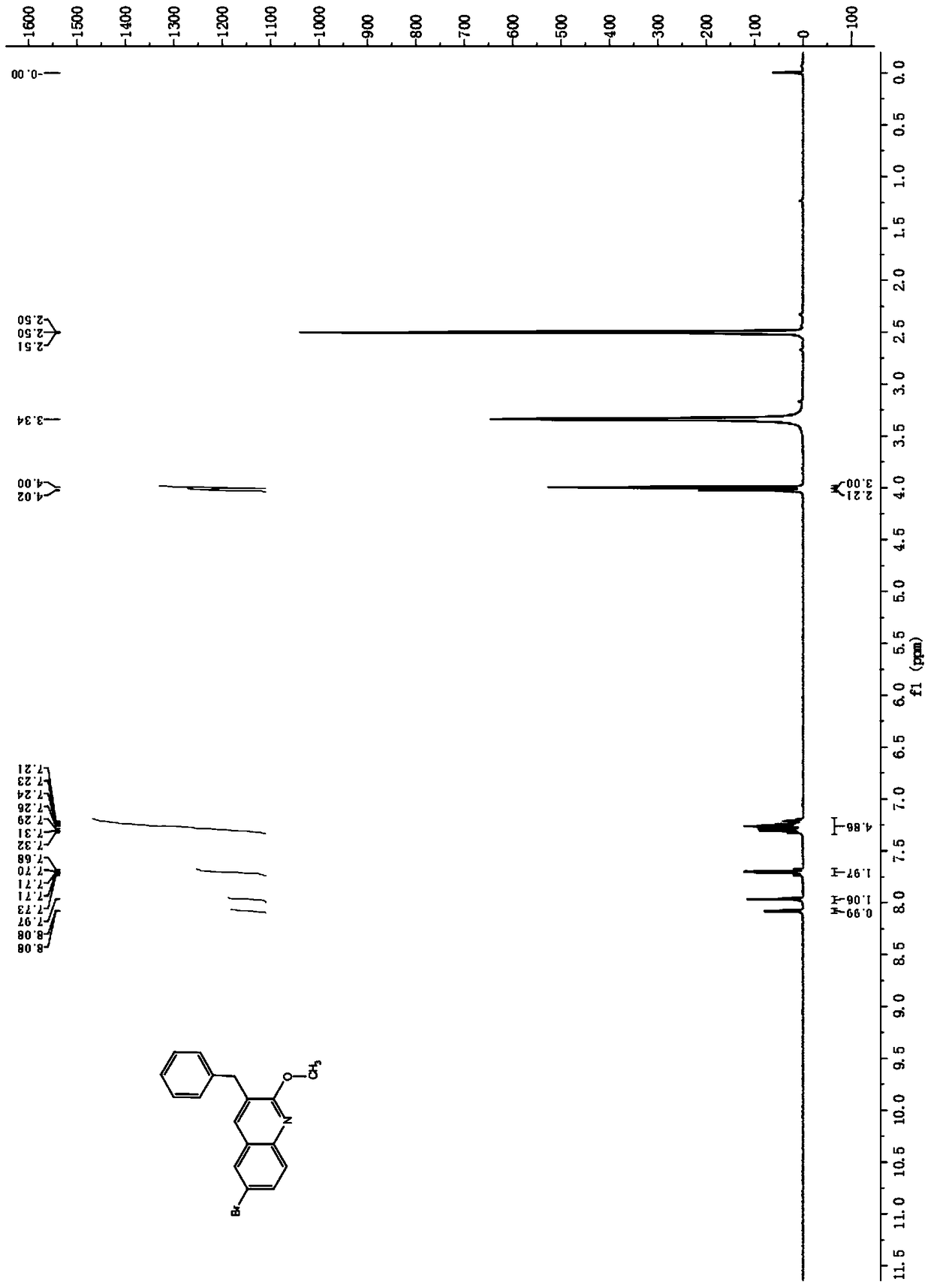
[0071] Product 3-benzyl-6 bromo-2-methoxyquinoline (see the attached figure 1 ) and 3-dimethylamino-1...
Embodiment 2
[0075] Using diastereomer A instead of diastereomer B, the procedure of Example 1 was repeated (yield X: 83%, Y: 85%).
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com