Cu(I)-based metal organic framework, and preparation method and applications thereof
A technology of metal-organic frameworks and organic ligands, applied in the direction of 1/11 organic compounds without C-metal bonds, organic chemistry, copper organic compounds, etc., to achieve low detection limits, ensure accuracy, and highly selective effects
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0060] 1. Preparation of organic ligand L
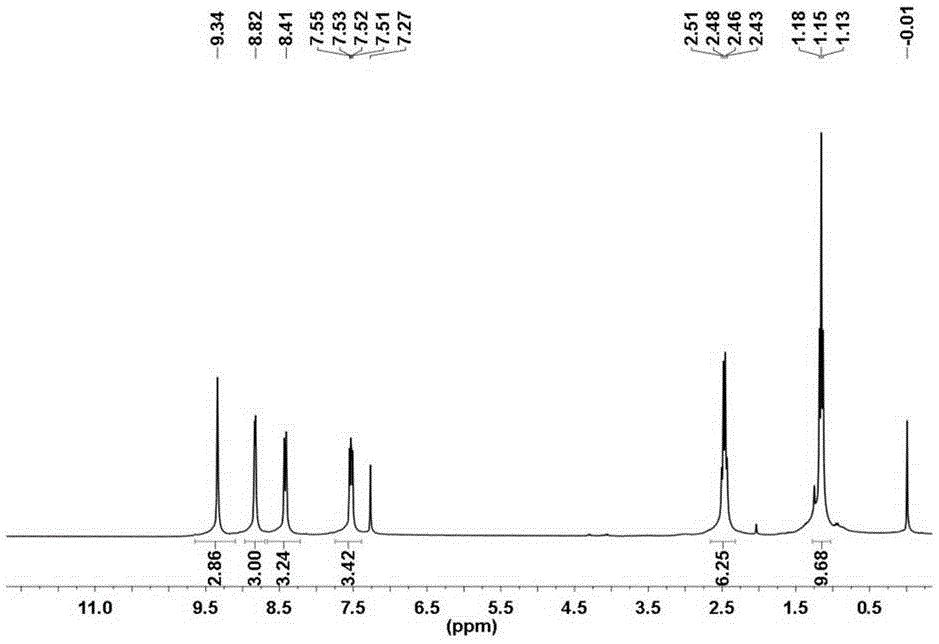
[0061] Add 4.41 g of 5-(3-pyridyl) tetrazole to 3.48 g of 2,4,6-triethyl-1,3,5-trimesoyl chloride, 10 ml of anhydrous pyridine, and react at 120° C. for 2 h. After the system was cooled, 100 ml of water was added, and the dark crude product was obtained by suction filtration, dried, and column chromatography (ethyl acetate) gave 3.1 g of light yellow powder with a yield of 53%. we pass 1 The compound was characterized by H NMR and IR, and the results are shown in Figure 4 and Figure 5 . 2,4,6-Triethyl-1,3,5-trimesoyl chloride References C.-W.Zhao, J.-P.Ma, Q.-K.Liu, Y.Yu, P.Wang ,Y.-A.Li,K.Wang and Y.-B.Dong,Green Chem.,2013,15,3150-3154.
[0062]
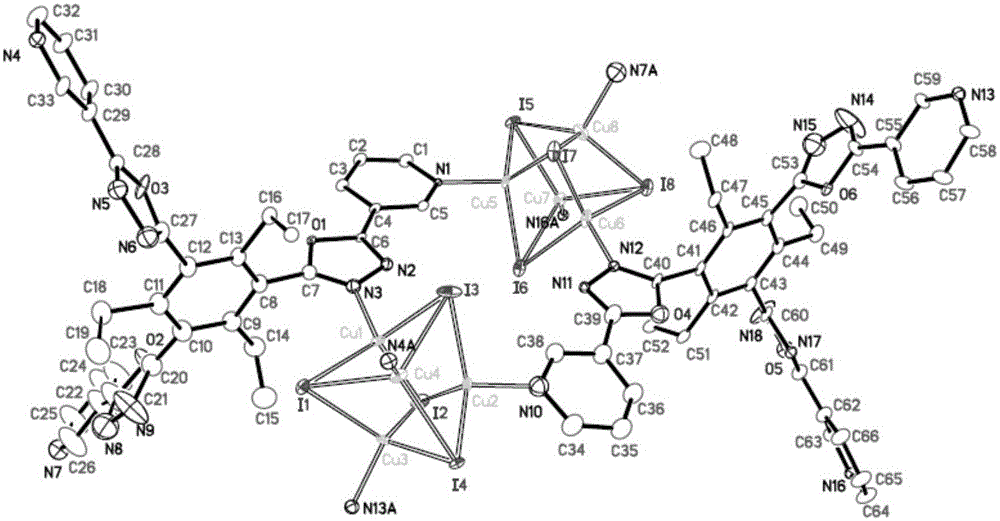
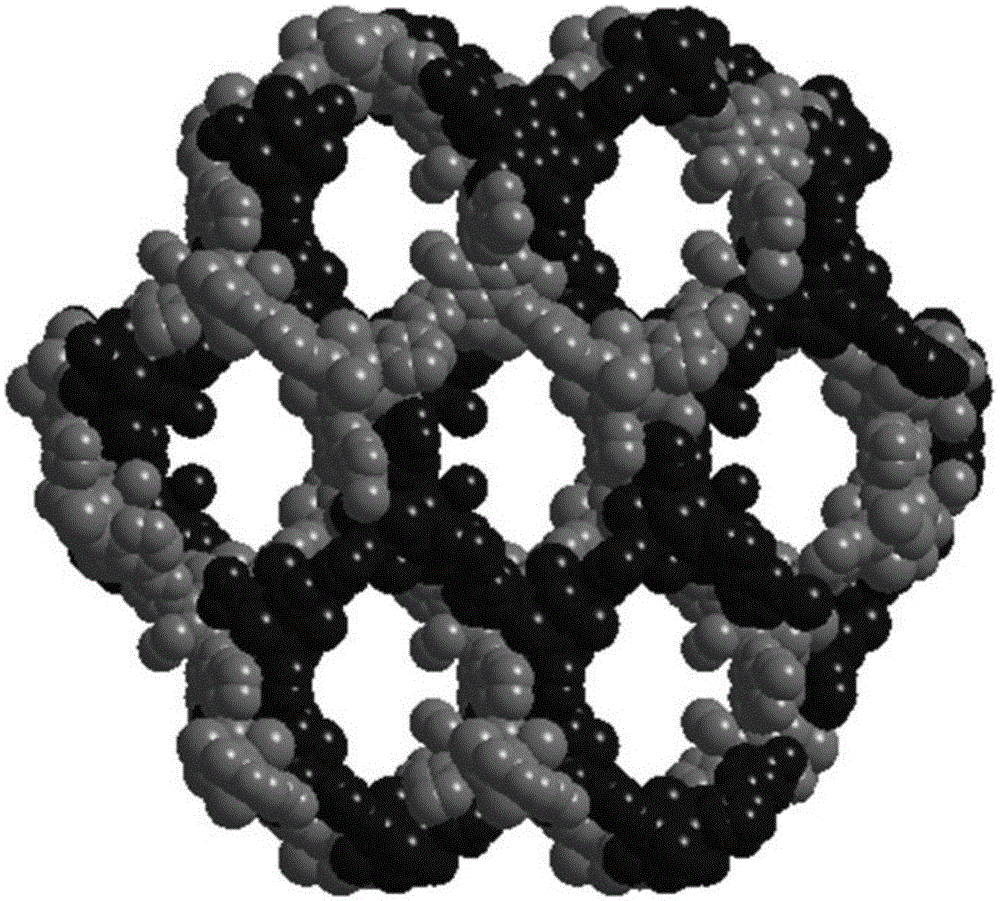
[0063] 2. Synthesis of CuI-MOF
[0064] Dissolve 30mg of organic ligand L and 40mg of CuI in 10mL of acetonitrile respectively, mix the two evenly, and let stand at 10°C for 15 hours to obtain 53mg of metal organic framework CuI-MOF with a yield of 77% (based on L).
[0065] W...
Embodiment 2
[0082] 1. Preparation of organic ligand L
[0083] Add 3.88 g of 5-(3-pyridyl) tetrazole to 2.80 g of 2,4,6-triethyl-1,3,5-trimesoyl chloride, 9.5 mL of anhydrous pyridine, and react at 110°C for 3.5 h. After the system was cooled, 100 mL of water was added, and the dark crude product was obtained by suction filtration, dried, and column chromatography (ethyl acetate) gave 3.0 g of light yellow powder with a yield of 52%.
[0084] 2. Synthesis of CuI-MOF
[0085] Dissolve 27mg of organic ligand L and 30mg of CuI in 9mL of acetonitrile, mix the two evenly, and let stand at 30°C for 12 hours to obtain 51mg of metal organic framework CuI-MOF with a yield of 76% (based on L).
[0086] 3. I 2 Synthesis of CuI(Cl)-MOF
[0087] The CuI-MOF crystal prepared in Example 2 was placed in a 50mL container with an HCl (g) concentration of 200ppm at 25°C, taken out after 14min, and left to stand in air for 150min to obtain I 2 CuI(Cl)-MOF.
[0088] 4. Synthesis of micro-CuI-MOF
[0089] ...
Embodiment 3
[0091] 1. Preparation of organic ligand L
[0092] Add 5.51 g of 5-(3-pyridyl) tetrazole to 3.80 g of 2,4,6-triethyl-1,3,5-trimesoyl chloride, 11 mL of anhydrous pyridine, and react at 130° C. for 1.5 h. After the system was cooled, 100 mL of water was added, the dark crude product was obtained by suction filtration, dried, and column chromatography (ethyl acetate) gave 3.2 g of a light yellow powder with a yield of 55%.
[0093] 2. Synthesis of CuI-MOF
[0094] Dissolve 35mg of organic ligand L and 42mg of CuI in 12mL of acetonitrile respectively, mix the two evenly, and let stand at 10°C for 15 hours to obtain 55mg of metal organic framework CuI-MOF with a yield of 78% (based on L).
[0095] 3. I 2 Synthesis of CuI(Cl)-MOF
[0096] The CuI-MOF crystal prepared in Example 2 was placed in a 50mL container with an HCl (g) concentration of 150ppm at 25°C, taken out for 10min, and left to stand in air for 100min to obtain I 2 CuI(Cl)-MOF.
[0097] 4. Synthesis of micro-CuI-M...
PUM
| Property | Measurement | Unit |
|---|---|---|
| particle diameter | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com