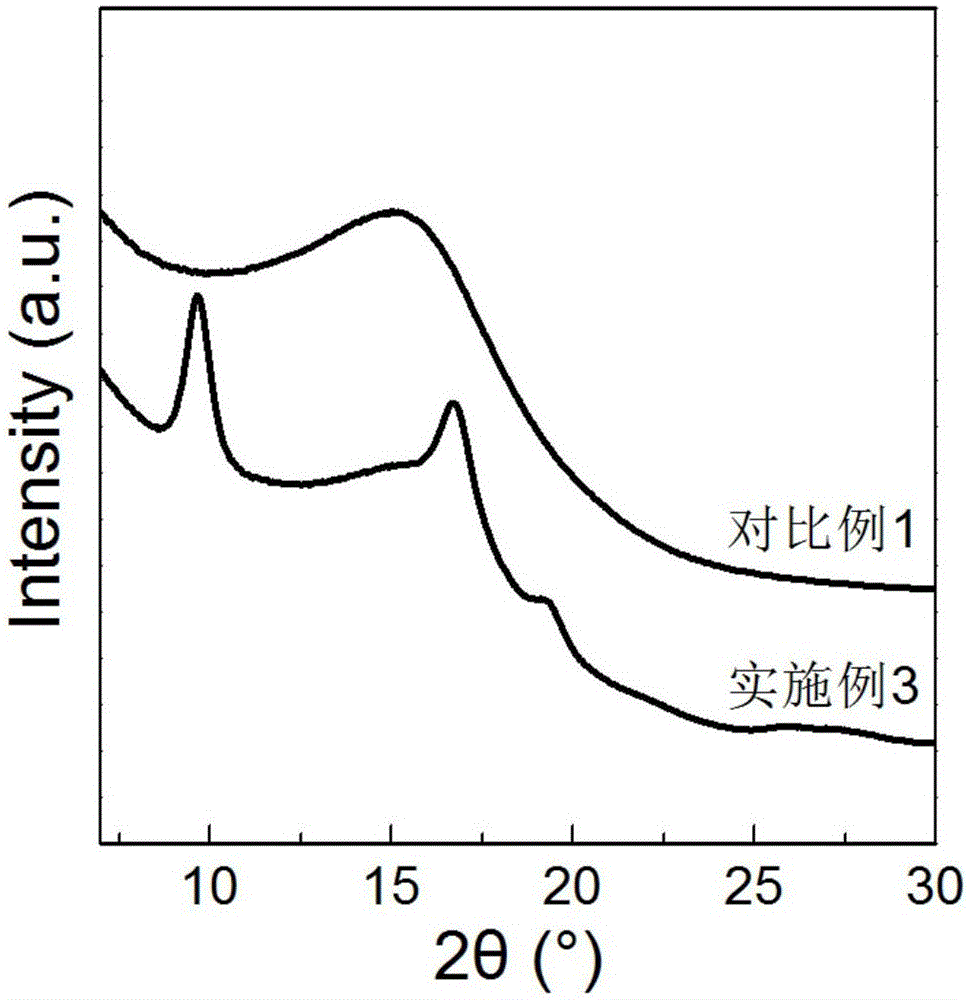
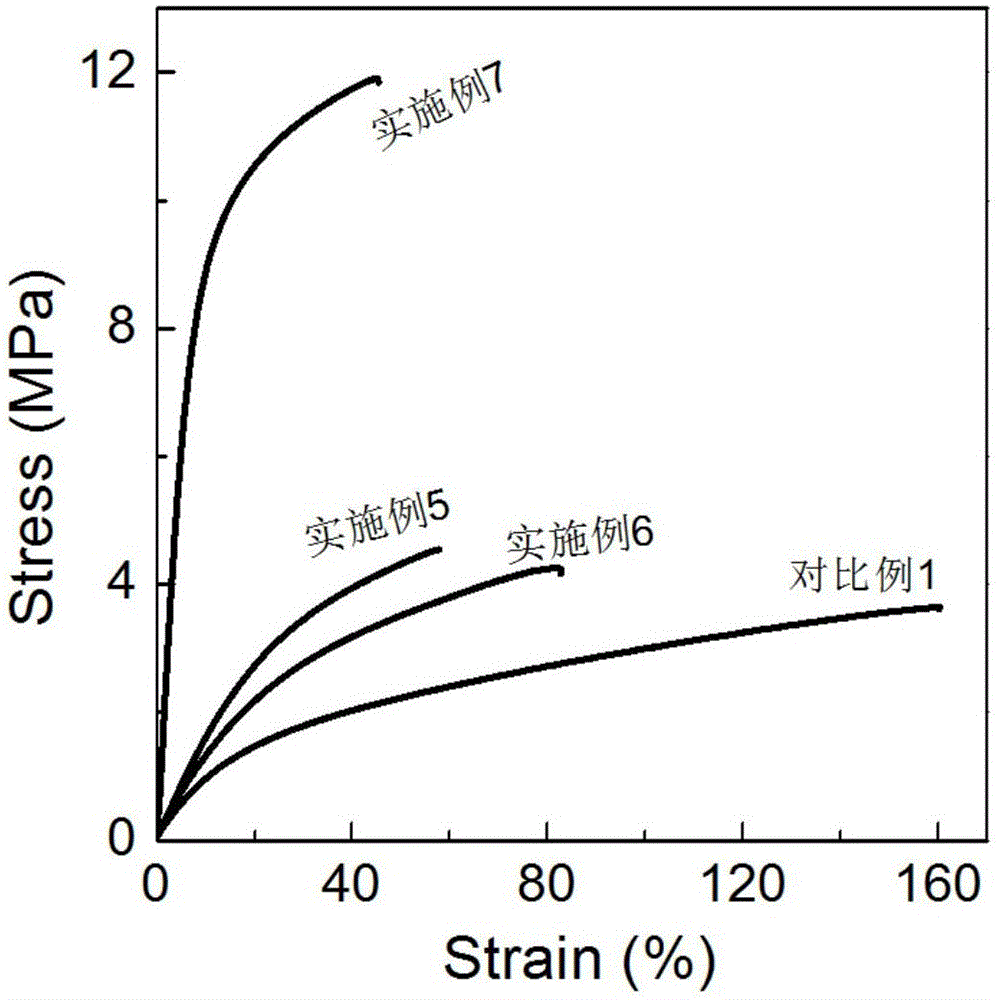
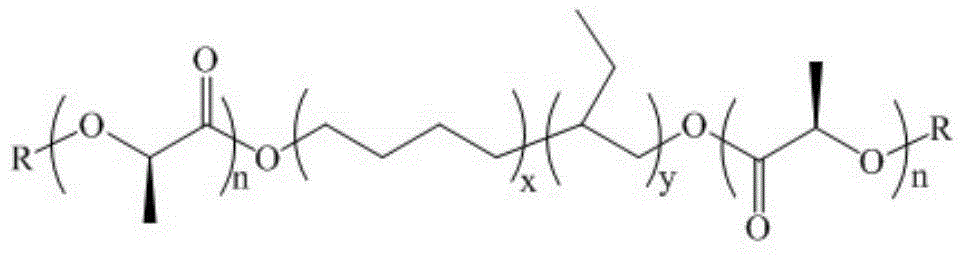
Steric compound crystal controlled polylactic acid/hydrogenated polybutadiene supermolecular elastomer
A technology of hydrogenated polybutadiene and stereocomplex crystallization, which is applied in the field of polymer thermoplastic elastomers, can solve the problems of poor processability, low modulus and heat resistance, and low crystallinity, and achieve high melting point and low pollution , high strength and modulus effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment Construction
[0026] The present invention will be further described in detail below in conjunction with the accompanying drawings and specific embodiments.
[0027] In the examples and comparative examples, the double-terminated hydroxyl-terminated PEB used was purchased from Sartomer, USA. The L-lactide and D-lactide used were purchased from Praque, recrystallized in ethyl acetate to remove impurities, and dried in vacuum at 60°C to constant weight.
[0028] UPy (UPy-NCO) containing isocyanate functional group is prepared according to the method described in the literature (So ntjens et al. Macromolecules, 2008, 41:5703-5708), the specific method is as follows: 2-amino-4-hydroxyl-6 -Methylpyrimidine (20.0g) was added to a 500ml three-necked flask, vacuumed at 50°C for 1h, protected by argon, and 180.3g of hexamethylene diisocyanate (HDI) and 6.5g of methylpyrrolidone were added as catalysts, wherein HDI The molar number is 7 times of that of 2-amino-4-hydroxyl-6-methylpyrimidine, and the...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com