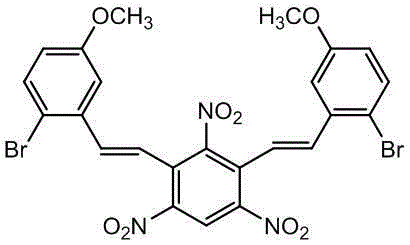
1,3-bis(2-bromo-5-methoxylstyryl)-2,4,6-trinitrobenzene and preparation method thereof
A technology of methoxystyrene and trinitrobenzene, applied in 1, can solve the problems of limited compounds, side-chain side reactions, poor regioselectivity, etc., and achieve the effect of good symmetry
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0017] Example 1: 1,3-bis(3-methoxystyryl)-2,4,6-trinitrobenzene (0.48g), liquid bromine (0.32g), H-Beta molecular sieve (0.05g), zinc oxide (0.05g) and dichloromethane (10mL) were added to the reactor, reacted at room temperature for 24h, filtered, extracted, and rotary evaporated to obtain 0.61g of an orange-yellow crude product, which was recrystallized with acetone and ethanol to obtain an orange-yellow pure product 0.59g, melting point 211.2-213.2°C, yield 96.7%.
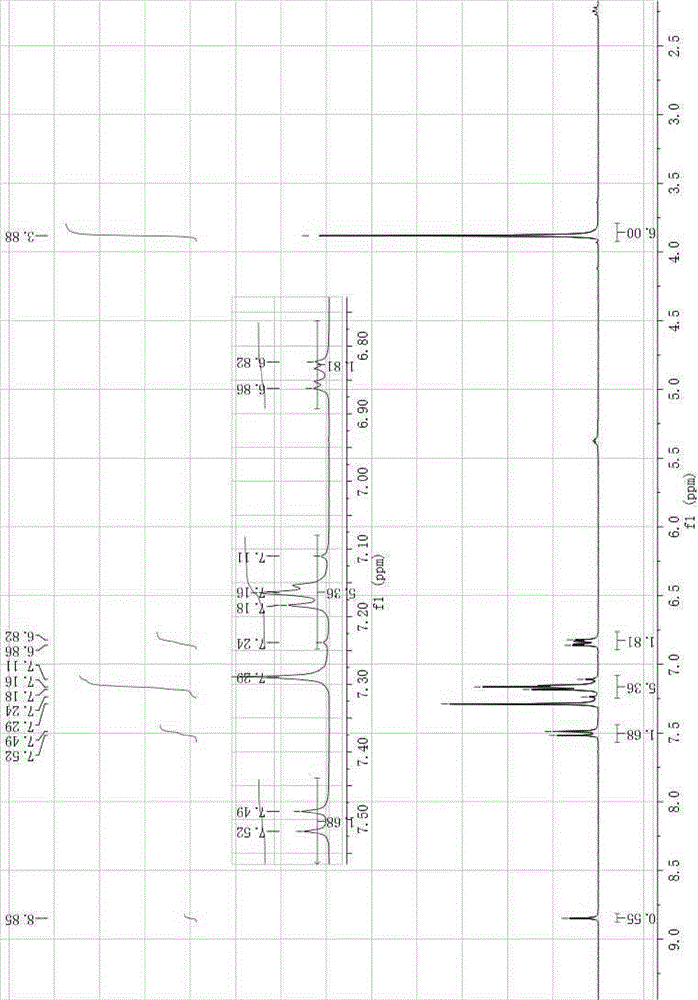
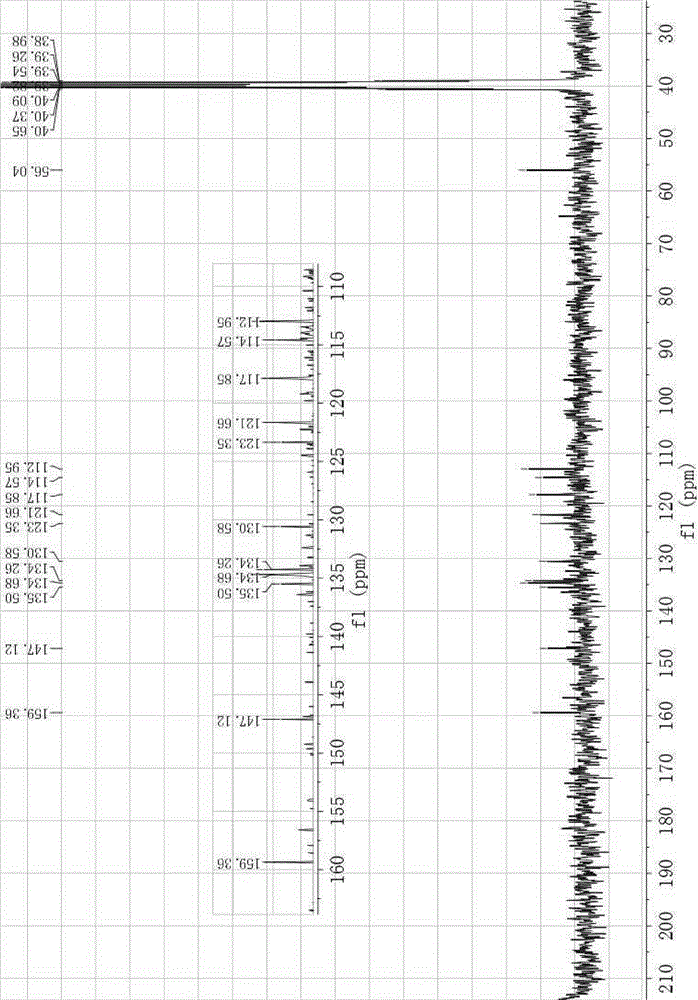
[0018] IR(KBr,ν,cm -1 ):3095,1634,1590,1539,1332,970; 1 HNMR (300MHz, CDCl 3 ),δ8.85(s,1H),7.52(d, J =8.7Hz,2H),7.18-7.15(m,6H),6.86-6.82(m,2H),3.88(s,6H); 13 CNMR (75MHz, DMSO-d 6 ), δ159.36, 147.12, 135.50, 134.68, 134.26, 130.58, 123.35, 121.66, 117.85, 114.57, 112.95, 56.04.
Embodiment 2
[0019] Example 2: 1,3-bis(3-methoxystyryl)-2,4,6-trinitrobenzene (0.48g), liquid bromine (0.32g), H-Beta molecular sieve (0.1g), zinc oxide (0.05g) and dichloromethane (10mL) were added to the reactor, reacted at room temperature for 24h, filtered, extracted, and rotary evaporated to obtain 0.61g of an orange-yellow crude product, which was recrystallized with acetone and ethanol to obtain an orange-yellow pure product 0.55g, the yield is 90.2%.
Embodiment 3
[0020] Example 3: 1,3-bis(3-methoxystyryl)-2,4,6-trinitrobenzene (0.48g), liquid bromine (0.32g), H-Beta molecular sieve (0.02g), zinc oxide (0.05g) and dichloromethane (10mL) were added to the reactor, reacted at room temperature for 24h, filtered, extracted, and rotary evaporated to obtain 0.53g of an orange-yellow crude product, which was recrystallized with acetone and ethanol to obtain an orange-yellow pure product 0.42g, yield 79.2%.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com