Method for preparing polysaccharide-ADH derivative by means of A-group meningococcal refined sugar
A meningococcal,-ADH technology, applied in the field of preparation of polysaccharide-ADH derivatives, can solve the problems of unfavorable protection of human health and the environment, achieve better derivation effect, ensure human health, and increase the effect of ADH derivation rate
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
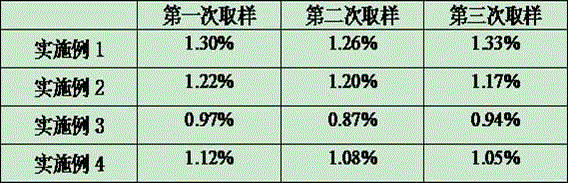
Embodiment 1
[0025] The method for preparing polysaccharide-ADH derivatives by utilizing group A meningococcal refined sugar comprises the following steps:
[0026] (1) Dissolve meningococcal refined sugar in water for injection to prepare a polysaccharide solution with a concentration of 5 mg / ml, and adjust the pH to 9.0 with sodium hydroxide;
[0027] (2) Dissolve CDAP with acetonitrile to form a CDAP solution with a concentration of 100mg / ml; then add CDAP solution to the polysaccharide solution according to the weight ratio of polysaccharide:CDAP=1:0.5, and stir at room temperature for 2-5 minutes to make a CDAP mixture ;
[0028] (3) Add ADH with 0.2mol / L NaHCO 3 Dissolve into an ADH solution with a concentration of 100mg / ml, add ADH solution to the CDAP mixture according to the weight ratio of polysaccharide: ADH=1:3.5, and stir at room temperature for 1 hour;
[0029] (4) Finally, filter with ultrafiltration membrane bag. The ultrafiltrate used in ultrafiltration membrane bag filt...
Embodiment 2
[0031] The only difference between this example and Example 1 is that the weight ratio of polysaccharide and CDAP in step (2) in this example is 1:0.6; the weight ratio of polysaccharide and ADH in step (3) is 1:4.
Embodiment 3
[0033] The difference between this embodiment and embodiment 1 is only that the proportion and concentration of each chemical substance in this embodiment are added to the reaction, and the specific settings are as follows:
[0034] The concentration of the polysaccharide solution in step (1) is 10 mg / ml, and the pH is adjusted to 9.0 with sodium hydroxide;
[0035] The concentration of CDAP solution in step (2) is 80mg / ml, polysaccharide:CDAP=1:0.7;
[0036] The concentration of ADH solution in step (3) is 80mg / ml ADH solution, polysaccharide: ADH=1:5.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com