2-aryl-1,3-dihydro-benzimidazol derivative as well as synthesis method and application thereof
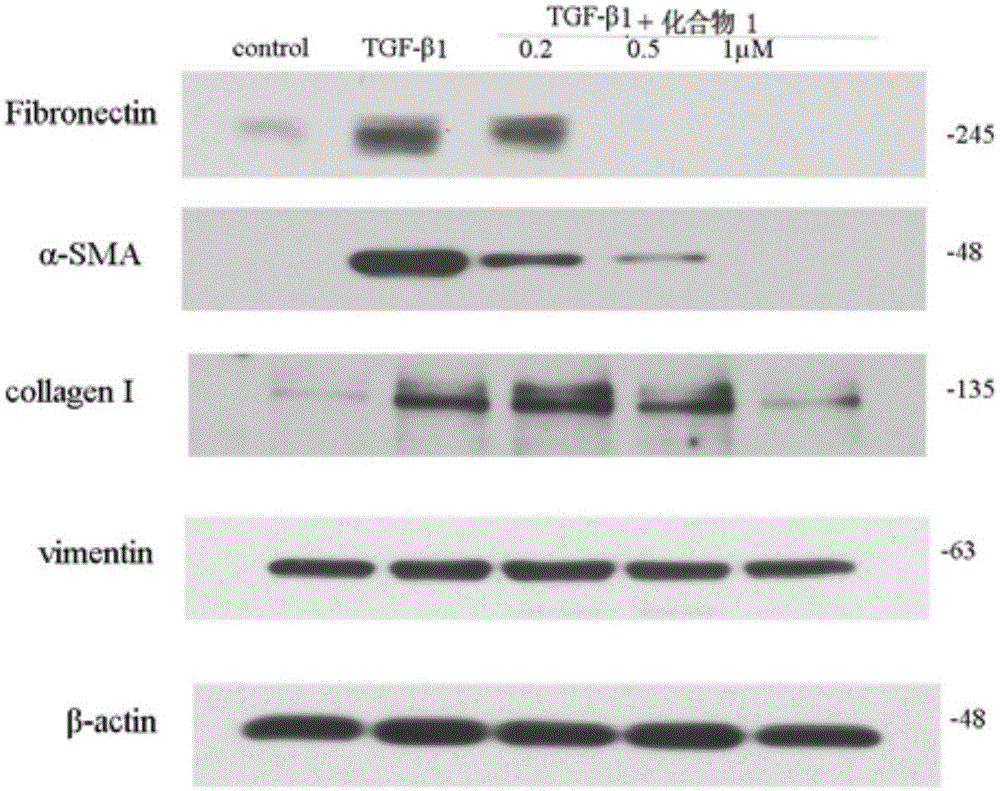
A synthesis method and drug technology, applied in the field of medicine, can solve the problems that have not been seen before, and achieve the effects of good medicinal value and significant anti-renal fibrosis activity.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0023] 1) Add 1.53g (10mmol) of 4-nitro-o-phenylenediamine, 1.75g (10mmol) of 2,4-dichlorobenzaldehyde, 50ml of anhydrous methanol into a 150ml round bottom flask, heat to reflux temperature and reflux for 8h . After the reaction was completed, it was cooled to room temperature, and the orange crude product was obtained by suction filtration.
[0024] 2) The crude product was subjected to silica gel column chromatography using a solvent composed of petroleum ether:dichloromethane=100:20 (volume ratio) as an eluent to obtain an orange solid product (2.85 g, yield 92%).
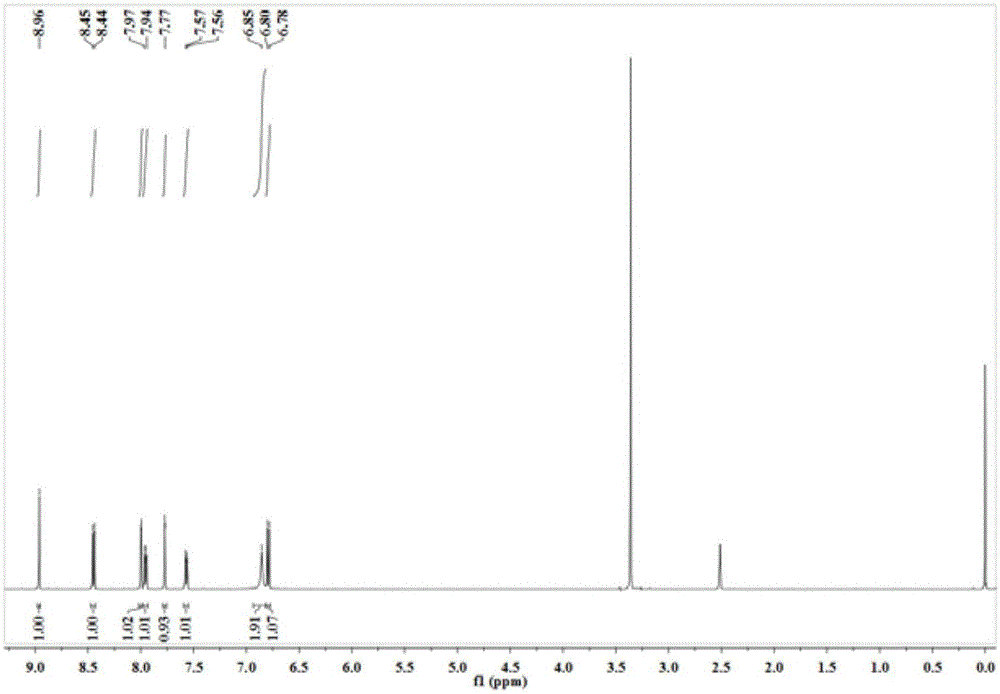
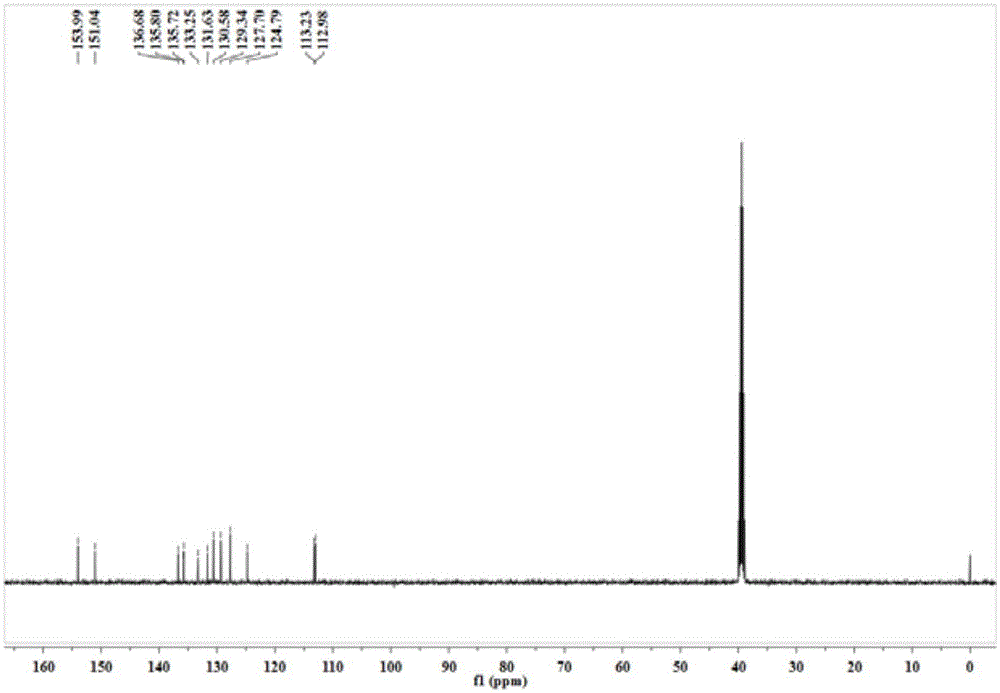
[0025] The resulting orange solid product is characterized by nuclear magnetic resonance, and its proton nuclear magnetic resonance spectrum and carbon spectrum are respectively as follows figure 1 and 2 shown.
[0026] 1 HNMR (500MHz, DMSO-d6) δ8.96(s, 1H), 8.45(d, J=8.5Hz, 1H), 8.00(d, J=2.5Hz, 1H), 7.95(dd, J=9.0, 2.5 Hz,1H),7.77(d,J=2.0Hz,1H),7.57(dd,J=8.5,1.7Hz,1H),6.85(s,2H),6.79(d,J=9.0Hz,1H). 13...
Embodiment 2
[0030] 1) Add 1.53g (10mmol) of 4-nitro-o-phenylenediamine, 0.88g (5mmol) of 2,4-dichlorobenzaldehyde, 50ml of absolute ethanol into a 150ml round bottom flask, heat to reflux temperature for 8h . After the reaction was completed, it was cooled to room temperature, and the orange crude product was obtained by suction filtration.
[0031] 2) The crude product was subjected to column chromatography using a solvent composed of petroleum ether:dichloromethane=100:15 (volume ratio) as an eluent to obtain an orange solid product (1.36 g, yield 88%).
[0032] The obtained orange solid product was characterized by NMR, and was determined to be 2-(2,4-dichlorophenyl)-5-nitro-1,3-dihydrobenzimidazole described in the present invention.
Embodiment 3
[0034] 1) Add 1.53g (10mmol) of 4-nitro-o-phenylenediamine, 1.75g (10mmol) of 2,4-dichlorobenzaldehyde, 25ml methyl alcohol and 25ml absolute ethanol into a 150ml round bottom flask and heat to Reflux temperature reflux reaction 6h. After the reaction was completed, it was cooled to room temperature, and the orange crude product was obtained by suction filtration.
[0035] 2) The crude product was subjected to column chromatography using a solvent composed of petroleum ether:dichloromethane=100:15 (volume ratio) as an eluent, and an orange solid product (2.48 g, yield 80%) was obtained.
[0036] The obtained orange solid product was characterized by NMR, and was determined to be 2-(2,4-dichlorophenyl)-5-nitro-1,3-dihydrobenzimidazole described in the present invention.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com