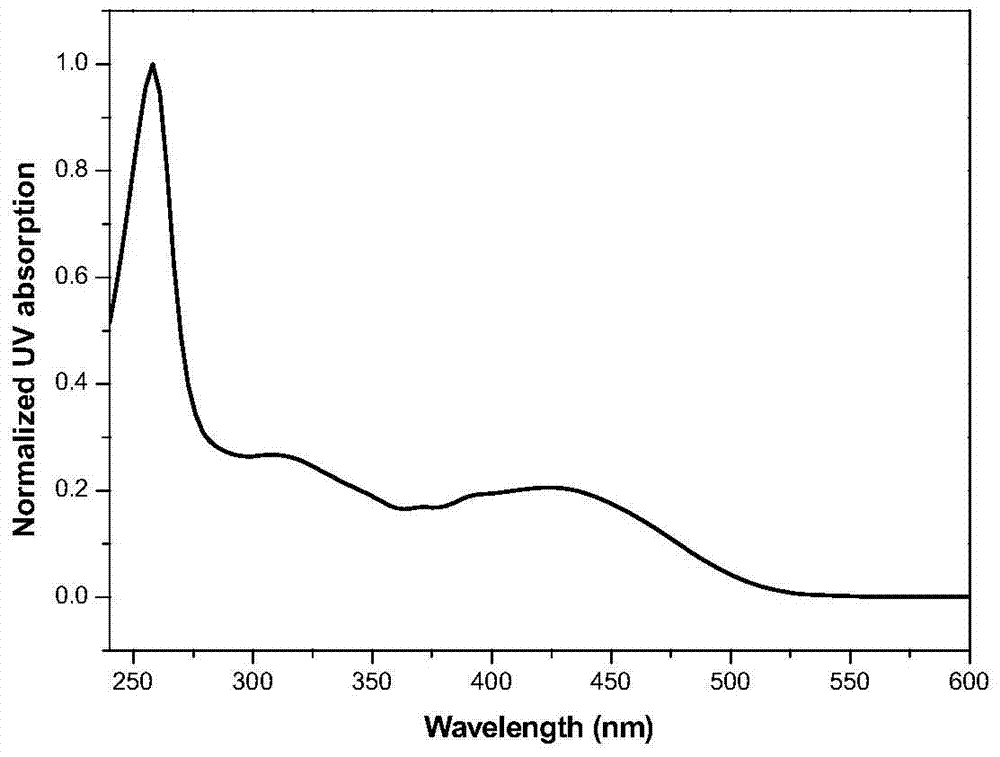
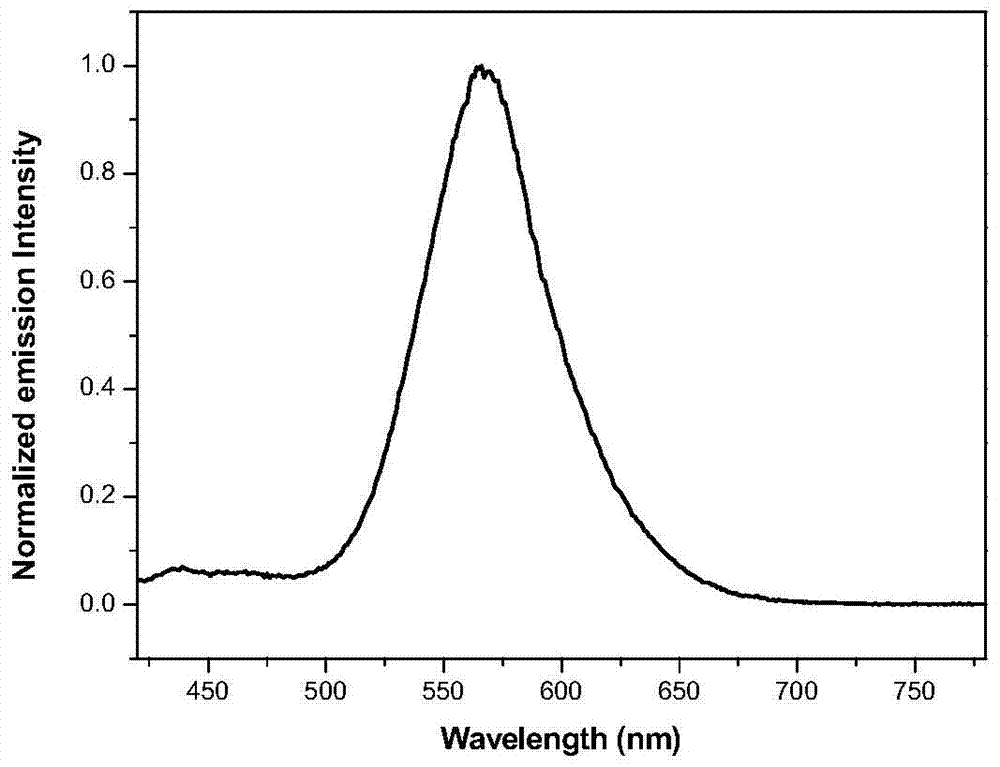
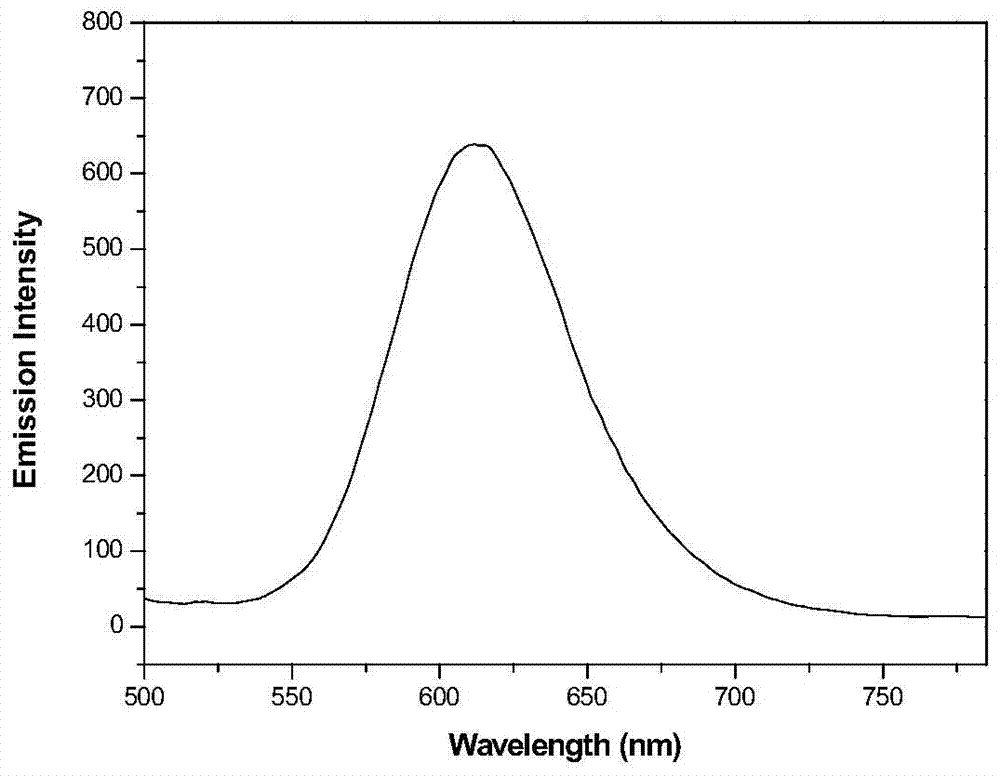
A multifunctional base pyridazinone compound, its application as an orange organic luminescent material and its preparation method
A technology based on pyridazinone and light-emitting materials, which is applied in the application and preparation of orange organic light-emitting materials, and in the field of multifunctional pyridazinone compounds, can solve problems that have not been reported, and achieve improved hole transport performance and effective Conducive to the effect of transmission and structural stability
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0053] Example 1: 4,5-dihydro-5-methyl-6-(2,3-bis(4-(9-anthracenyl)-1,3-butadienyl)-6-quinoxalinyl )-3 (2H) preparation of pyridazinone (I)
[0054] Synthesis of step a, 1,10-bis(9-anthracenyl)-1,3,7,9-decatetraene-5,6-dione (IV)
[0055] In a dry 100 ml round bottom flask, add 2,3-butanedione (0.01mol), 3-(9-anthracenyl)acrolein (0.02mol), and anhydrous methanol (50 ml), then rapidly Piperidine (0.5 mL) was added to the solution with stirring, and then stirred and refluxed for 6-8 hours. After the reaction was completed, it was cooled to room temperature, and a red solid was precipitated, which was filtered under reduced pressure, washed three times with absolute ethanol, recrystallized with a mixed solvent of ethanol-N,N-dimethylformamide, and dried in vacuo to obtain a red solid with a yield of 19%.
[0056] Step b, 4,5-dihydro-5-methyl-6-(2,3-di(4-(9-anthracenyl)-1,3-butadienyl)-6-quinoxalinyl) Synthesis of -3(2H)pyridazinone (I)
[0057] In a dry 100 ml round bottom ...
Embodiment 2
[0059] Example 2: 4,5-dihydro-5-methyl-6-(2,3-bis(4-(9-anthracenyl)-1,3-butadienyl)-6-quinoxalinyl )-3 (2H) preparation of pyridazinone (I)
[0060] According to the method of Example 1, it is obtained by two-step reaction, the difference is that in step (2), 1,10-di(9-anthracenyl)-1,3,7,9-decatetraene-5 , the molar ratio of 6-diketone and 6-(3,4-diaminophenyl)-5-methyl-4,5-dihydropyridazin-3(2H)-one is 1:1.1, and the yield is 68%.
Embodiment 3
[0061] Example 3: 4,5-dihydro-5-methyl-6-(2,3-bis(4-(9-anthracenyl)-1,3-butadienyl)-6-quinoxalinyl )-3 (2H) preparation of pyridazinone (I)
[0062] By the method of embodiment 1, obtain by two-step reaction, difference is, step (2) is carried out by microwave radiation method:
[0063] Add 1,10-bis(9-anthracenyl)-1,3,7,9-decatetraene-5,6-dione (0.001mol) and 6-(3, 4-Diaminophenyl)-5-methyl-4,5-dihydropyridazin-3(2H)-one (0.001mol), and 30mL of anhydrous acetic acid, stir well, put in microwave oven, The reaction is carried out under fire radiation, and the middle is taken out and stirred several times, and the reaction time is 10 to 15 minutes. After the reaction was completed, cool to room temperature, pour the reaction solution into ice water under rapid stirring, adjust the pH to 7 with 10% aqueous sodium hydroxide solution, filter the obtained solid under reduced pressure, wash with water three times, and dry at room temperature. Recrystallized from a mixed solvent of ...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com