Composition 67083001030512 and application of composition 67083001030512 to drug for resisting liver fibrosis
A liver fibrosis and composition technology, applied in the fields of organic synthesis and medicinal chemistry, can solve the problems of lack of clinical treatment methods
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
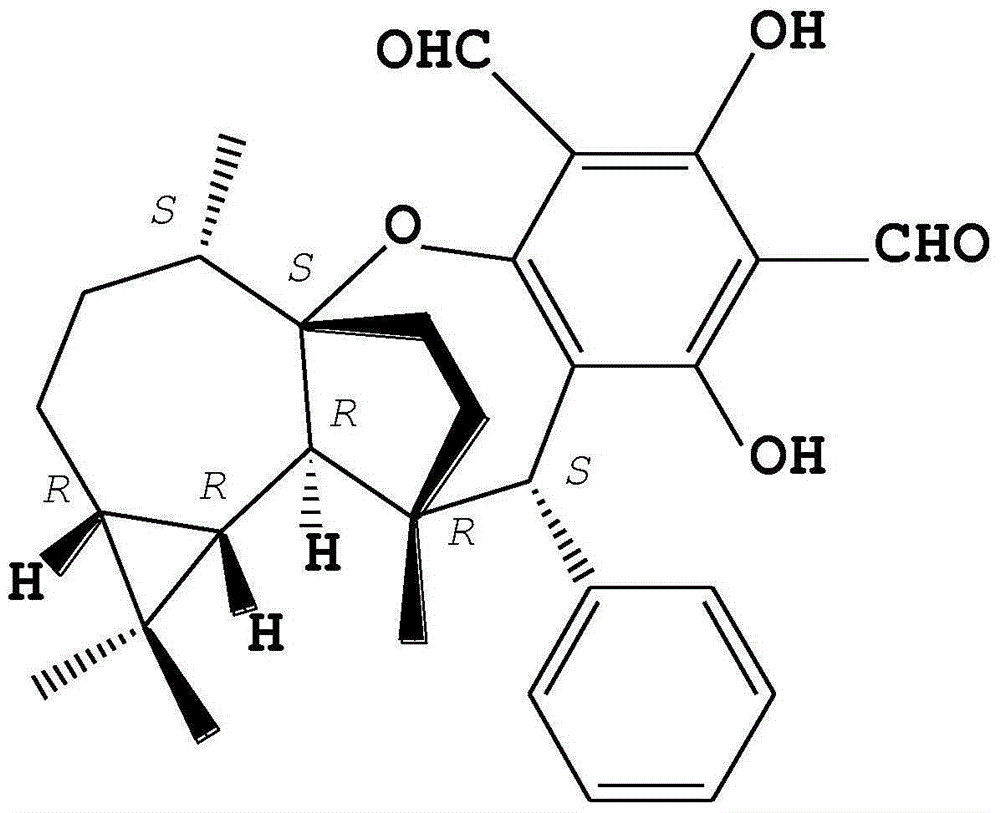
[0015] The preparation of embodiment 1 compound PsiguadialA
[0016] The preparation method of the compound PsiguadialA (I) refers to the method published by Meng Shao et al. (Meng Shao et al., 2010. Psiguadials A and B, Two Novel Meroterpenoids with Unusual Skeletons from the Leaves of Psidiumguajava. Organic Letters 12 (2010) 5040-5043).
[0017]
[0018]
Embodiment 2
[0019] The synthesis of the O-bromoethyl derivative (II) of embodiment 2PsiguadialA
[0020] Compound I (474 mg, 1.00 mmol) was dissolved in 20 mL of benzene, tetrabutylammonium bromide (TBAB) (0.16 g), 1,2-dibromoethane (7.520 g, 40.00 mmol) and 12 mL of 50% sodium hydroxide solution. The mixture was stirred at 35 °C for 8 h. After 8 hours, the reaction solution was poured into ice water, extracted twice with dichloromethane immediately, and the organic phase solutions were combined. Then the organic phase solution was washed with water and saturated brine three times successively, then dried with anhydrous sodium sulfate, and finally concentrated under reduced pressure to remove the solvent to obtain a crude product. The crude product was purified by silica gel column chromatography (mobile phase: petroleum ether / acetone=100:0.5, v / v), the brown concentrated elution band was collected and the solvent was evaporated to obtain a brown powder of Compound II (502mg, 73%) . ...
Embodiment 3
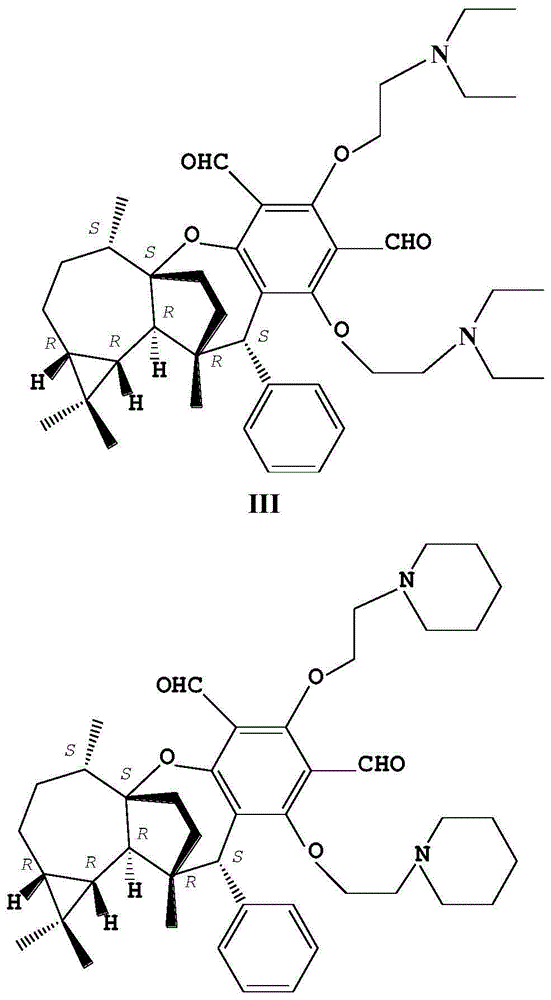
[0025] The synthesis of the O-(diethylamino) ethyl derivative (III) of embodiment 3PsiguadialA
[0026] Compound II (344mg, 0.5mmol) was dissolved in 20mL of acetonitrile, anhydrous potassium carbonate (690mg, 5.0mmol), potassium iodide (168mg, 1.0mmol) and diethylamine (2920mg, 40mmol) were added thereto, and the mixture was heated to reflux for 9h . After the reaction, the reaction solution was poured into ice water, extracted 4 times with an equal amount of dichloromethane, and the organic phases were combined. The combined organic phases were successively washed with water and saturated brine, dried over anhydrous sodium sulfate, and concentrated under reduced pressure to remove the solvent to obtain a crude product. The crude product was purified by silica gel column chromatography (mobile phase: petroleum ether / acetone=100:1.5, v / v), the yellow concentrated elution band was collected and the solvent was evaporated to obtain a yellow powder of compound III (231.8mg, 69% ...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com