A compound targeting ubiquitination to degrade Smad3
A compound and ubiquitination technology, applied in the direction of peptides, etc., can solve problems that are not targets of anti-fibrosis therapy
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0034] Embodiment 1: The construction method of the compound targeting ubiquitination and degrading Smad3 of the present invention (the compound described here is called Protac)
[0035] 1. The method for constructing the compound Protac targeting ubiquitination and degrading Smad3 according to the present invention
[0036] ① Determination of Smad3 active site and preparation of Smad3 protein;
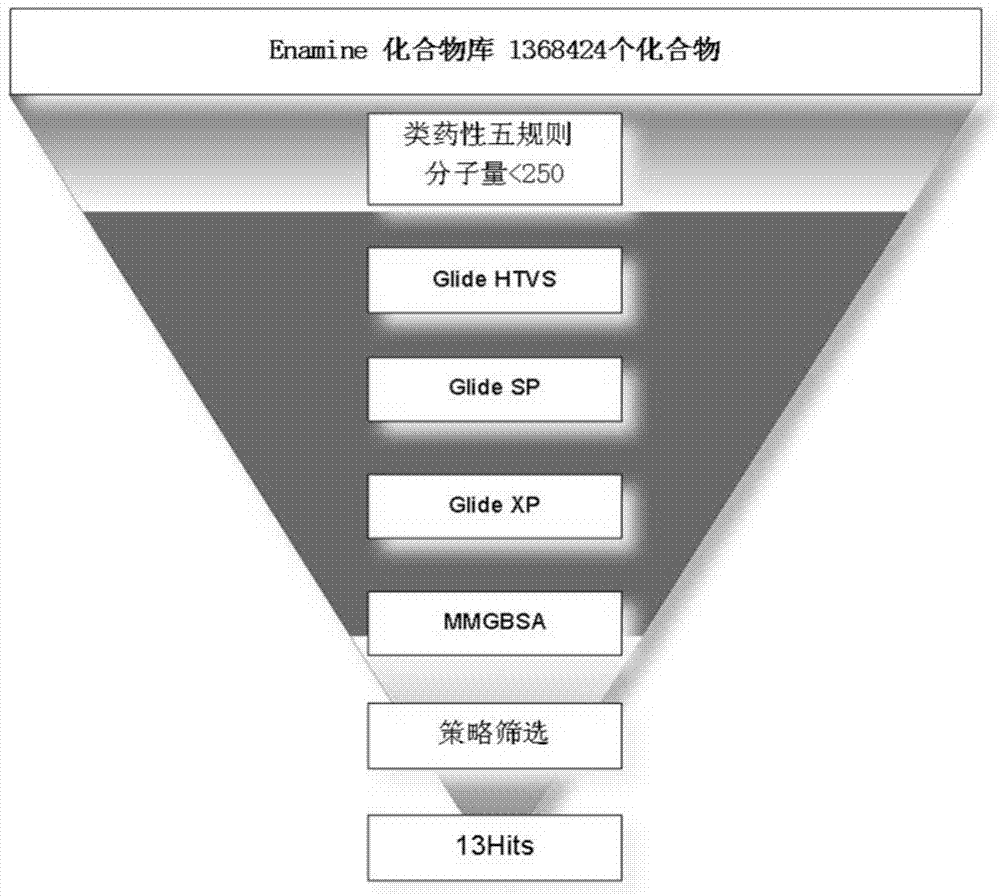
[0037] ② Enamine compound library preparation;
[0038] ③Glide step-by-step screening based on molecular docking;
[0039] ④ Hits results and analysis;
[0040] ⑤ Further screening of small molecules by Surface Plasmon Resonance (SPR) technology;
[0041] ⑥Construction and chemical synthesis of Protac;
[0042] ⑦Verification of Protac peptide structure.
[0043] 2. Construction results
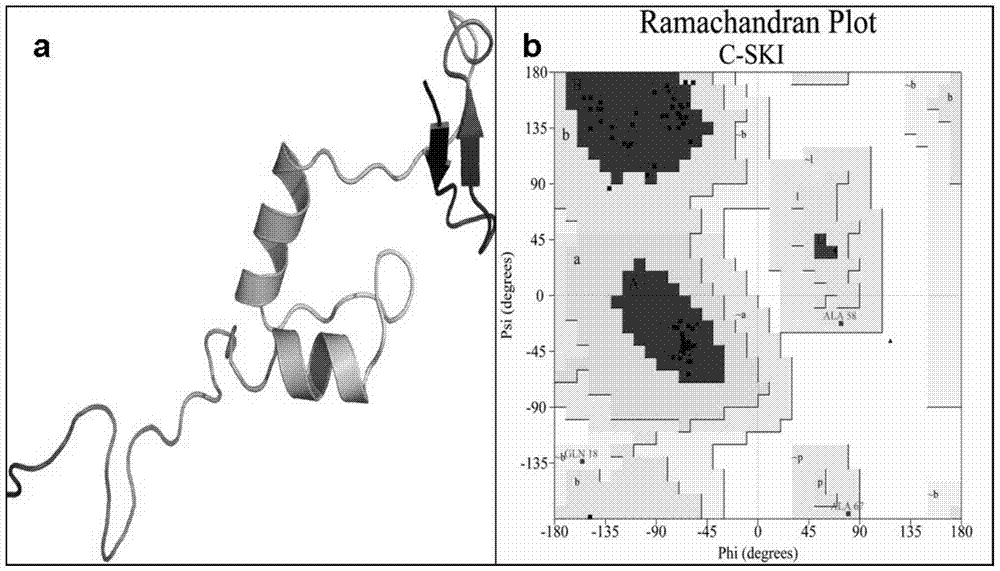
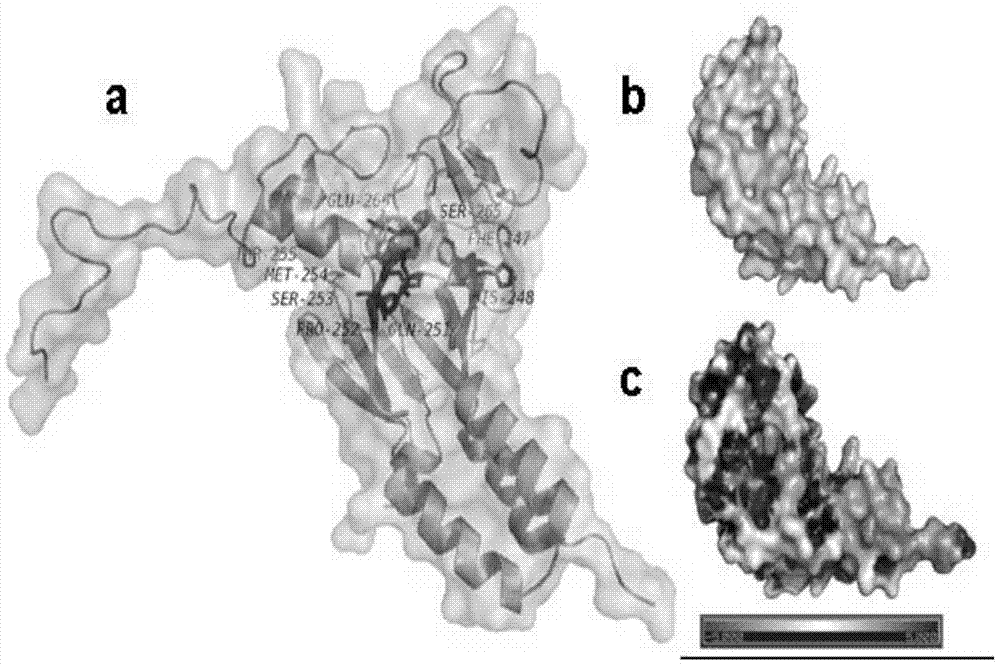
[0044] (1) Determination and preparation of the active site of Smad3
[0045] The establishment of the active site of Smad3 is the prerequisite for molecular simulation. The ROC1-SCFFbw1a comp...
Embodiment 2
[0087] Example 2: Verification of the degradation of Smad3 by targeting the ubiquitination pathway of the compounds targeting ubiquitination and degradation of Smad3 according to the present invention
[0088] Cell culture: DMEM medium for human renal carcinoma cell line (ACHN) and rat normal renal fibroblast cell line (NRK-49F), human renal clear cell adenocarcinoma cell line (786-0) and human glomerular mesangium 1640 medium for cell line (HMC), both containing 10% calf serum, 50×10 3 U / L penicillin and 50mg / L streptomycin at 37°C, 5% CO 2 Under normal subculture conditions.
[0089] Western detection of VHL protein expression: Collect 786-0, ACHN, NRK-49F and HMC cells in a 100mm cell culture dish, rinse twice with PBS on ice, add 1ml RIPA cell lysate (Millipore, USA), and lyse on ice for 20min , centrifuge at 13000 rpm for 15 min, take 50 μl of supernatant, add 25 μl of 3×SDS loading buffer and boil for 8 min. Centrifuge at 13,000rpm for 15min, take 20 μl of the superna...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com