Method for determining content of drug components in mixture drug
A technology of mixing drugs and mass percentage content, which is applied in the field of terahertz time-domain spectroscopy detection, can solve the problems of high professional technical requirements, low detection limit, expensive instruments, etc., and achieve the goals of avoiding harmful ionization, multiple data and accuracy Effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0085] (1) Establish a test model and detect the content of each drug component in the mixed drug sample to be tested
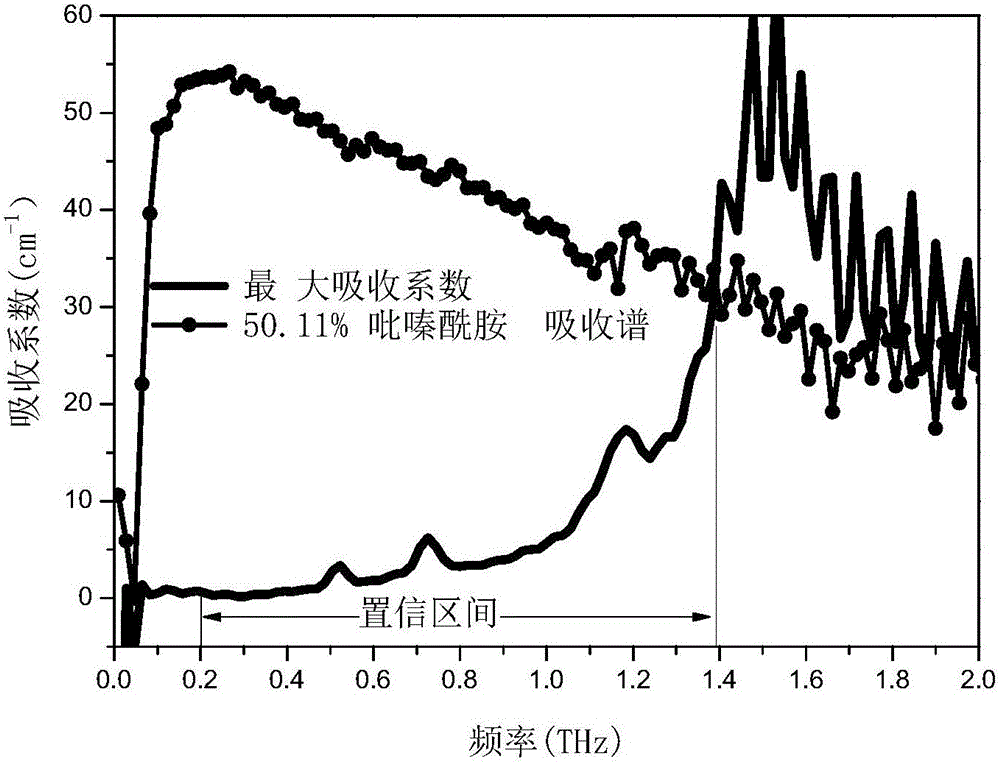
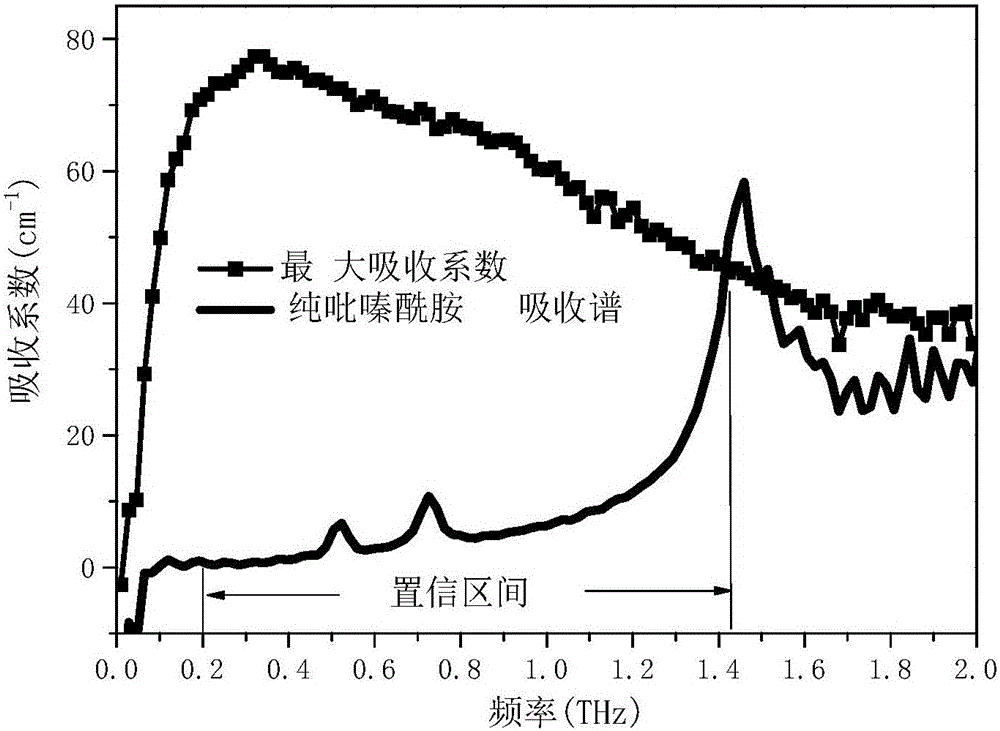
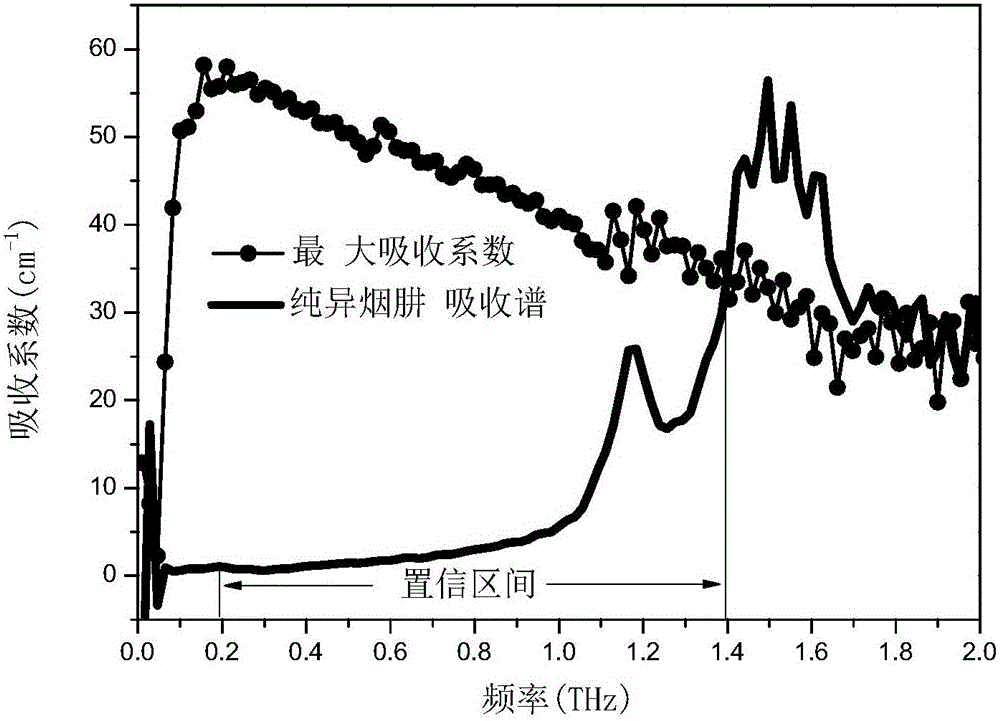
[0086] In this embodiment, the mixed drug is a mixture of pyrazinamide and isoniazid.
[0087] A mathematical method is used to establish a test model for the corresponding relationship between the content of pyrazinamide in the mixed medicine and the effective optical parameters of the mixed medicine. The specific method is as follows:
[0088] a. Preparation of standard samples
[0089] Weighed 16 groups of pure pyrazinamide and pure isoniazid with different masses respectively, and prepared 16 standards. The mass percentages of pyrazinamide in each standard are different, being 0%, 2.00%, 5.00%, 8.94%, 15.30%, 19.94%, 25.00%, 29.88%, 40.99%, 50.11%, 70.10% %, 74.97%, 80.03%, 85.00%, 88.98%, 100%. Wherein, the mass percentage composition of pyrazinamide is 100% standard substance shows that the component in this standard substance is pure pyrazinamide a...
Embodiment 2
[0138] In this embodiment, the mixed medicine is formed by mixing pyrazinamide and isoniazid.
[0139] The method for measuring the content of pyrazinamide in the mixed medicine of the present embodiment is different from Example 1 as follows:
[0140] (i) The established test model is the corresponding relationship between the mass percentage content of pyrazinamide in the mixed drug and the effective refractive index of the mixed drug.
[0141] (ii) Refractive index formula: n ( ω ) = φ ( ω ) ω d + 1
[0142] In the above formula, φ(ω) is the phase difference between the signal of the measured sample (that is, the terahertz time-domain spectrum obtained by scanning the measured sample) and its reference signal (the terahertz time-domai...
PUM
| Property | Measurement | Unit |
|---|---|---|
| refractive index | aaaaa | aaaaa |
| refractive index | aaaaa | aaaaa |
| refractive index | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com