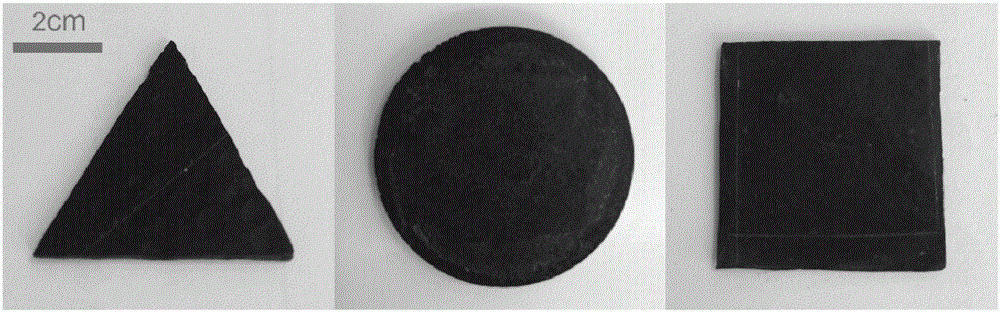
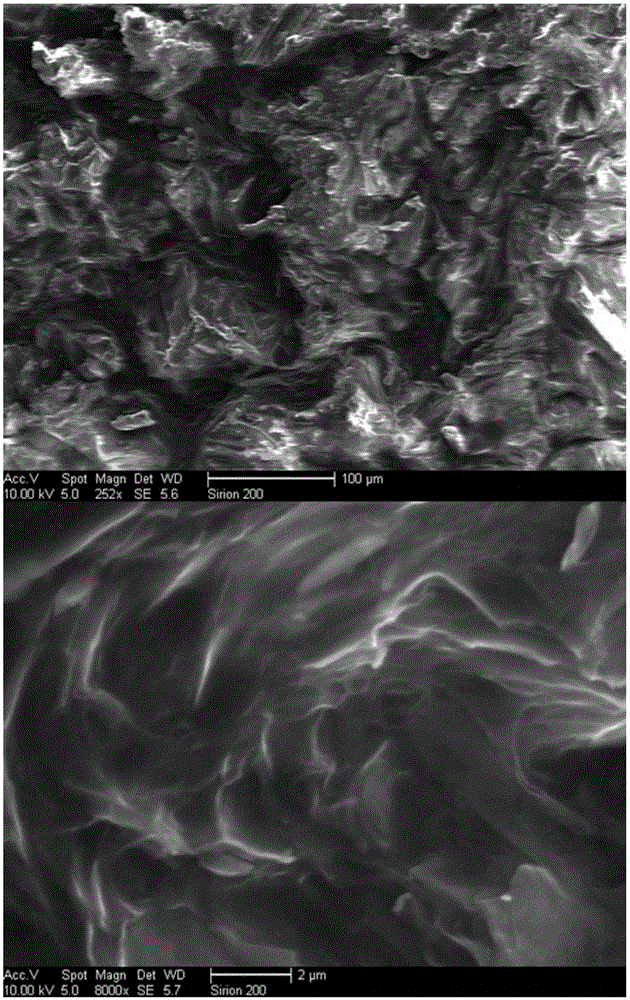
Energy-saving type three dimensional graphene skeleton composite phase change material with heat storage and release performances, and preparation method thereof
A composite phase change material, graphene skeleton technology, applied in the direction of heat exchange materials, chemical instruments and methods, can solve the problems of easy aging, easy leakage, low thermal conductivity, etc., to achieve good plasticity, prevent leakage, improve thermal conductivity, etc. guiding effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0035] The energy-saving three-dimensional graphene skeleton composite phase change material with heat storage and heat release performance in this embodiment is obtained by self-assembly of graphene and organic phase change material PEG2000, wherein the quality of organic phase change material PEG6000 is 9.5g, graphite The mass of alkenes is 0.5g.
[0036] In this embodiment, the energy-saving three-dimensional graphene skeleton composite phase change material with heat storage and heat release performance is prepared according to the following method:
[0037] Put 1.2g of graphite in 60mL of concentrated sulfuric acid with a mass concentration of 98%, 2gK 2 S 2 o 8 and 2gP 2 o 5 In the mixed solution, react at 85°C for 4.5 hours. After the reaction, dilute the reaction solution with 400mL deionized water, filter, wash and vacuum dry at 60°C in sequence to obtain pretreated graphite-graphite oxide;
[0038] In the graphite oxide of 100mg, add the concentrated sulfuric acid...
Embodiment 2
[0043] The preparation method of this example is the same as that of Example 1, except that the addition amount of the organic phase change material PEG6000 is 3 g and 1.5 g respectively.
[0044] After testing, when PEG6000 is used as the organic phase change material in Examples 1 and 2, the endothermic heat storage temperature of the obtained three-dimensional graphene skeleton composite phase change material is about 62-64°C.
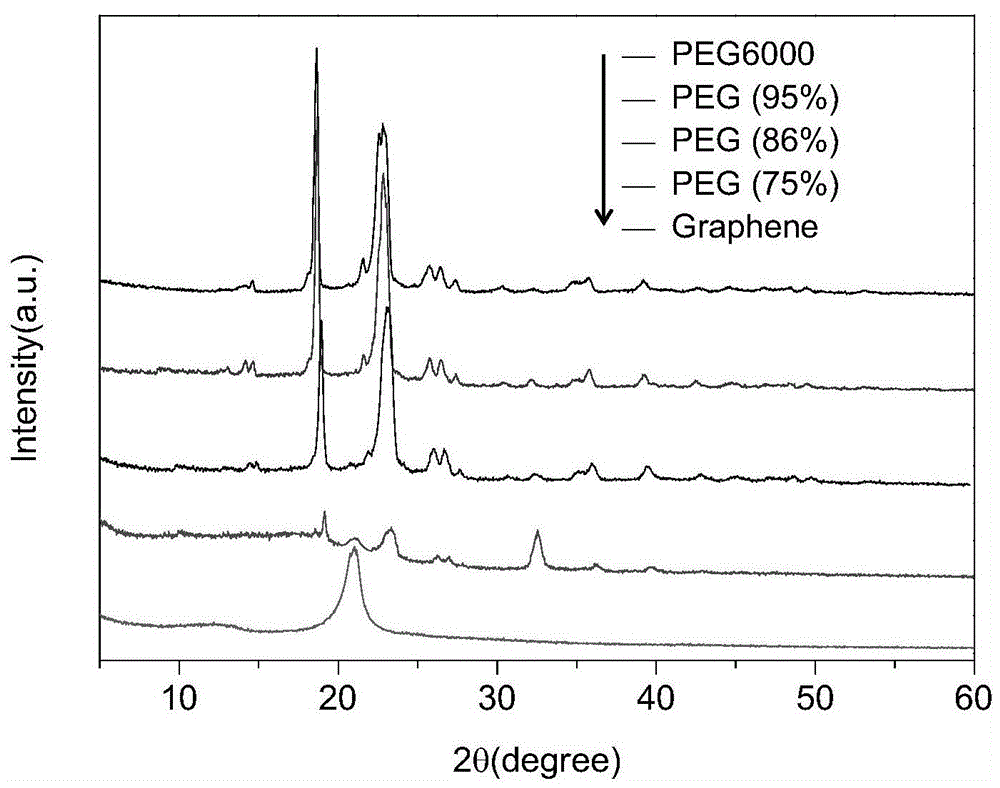
[0045] image 3 It is the XRD spectrum of the three-dimensional graphene skeleton composite phase change material of the different PEG6000 mass percentages of embodiment 1 and embodiment 2, and the XRD spectrum of pure PEG6000 and graphene, can find out from the XRD spectrum of graphene The diffraction peak at 20.9° is the characteristic peak of layered structure, so comparing with the XRD spectra of pure PEG6000 and graphene, it can be seen that graphene does not have this characteristic peak in phase change materials, so it is confirmed that graph...
Embodiment 3
[0049] The preparation method of this example is the same as that of Example 1, except that the organic phase change material is PEG2000, and the addition amounts are 9.5g, 3g and 1.5g respectively.
[0050] After testing, when PEG2000 is used as the organic phase change material in this embodiment, the endothermic heat storage temperature of the obtained three-dimensional graphene skeleton composite phase change material is about 41-46°C.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com