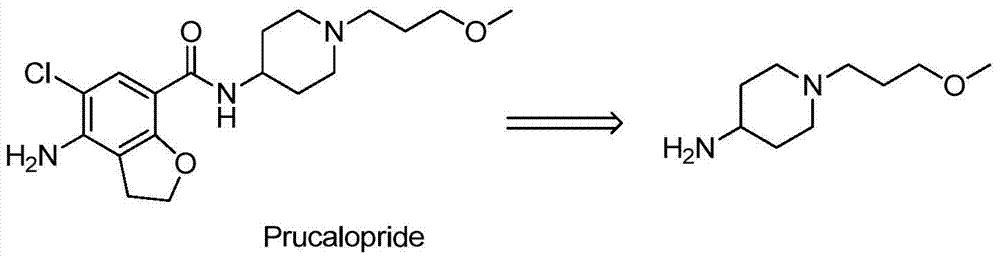
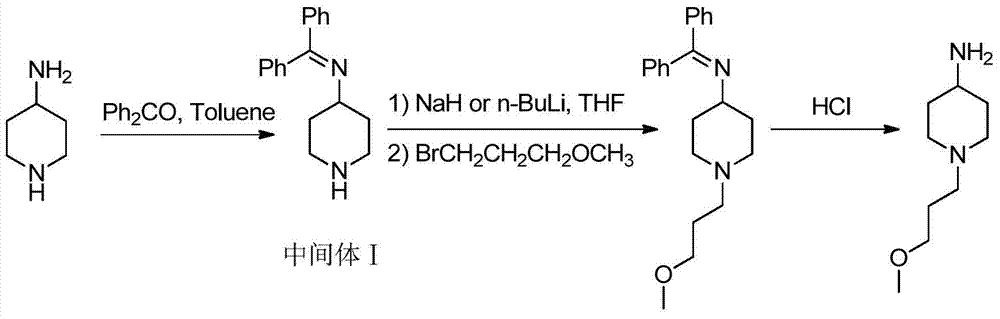
A method for preparing 1-(3-methoxypropyl) piperidine-4-amine
A technology of methoxypropyl and aminopiperidine, applied in the field of preparation of 1-piperidin-4-amine, which can solve the problems of limited universality and achieve the effect of simple operation
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0021] Synthesis of N-(dibenzylidene)piperidin-4-amine:
[0022] Add 4-aminopiperidine (50.1g, 0.5mol) and benzophenone (100.2g, 0.55mol) into a 500mL reaction flask with a separator, add 260mL of toluene and BF 3 -Et 2 After O (3.6g, 0.025mol), water was separated for 8-10 hours under reflux conditions. After the reaction was completed, the reaction solution was directly spin-dried, and the condensation dehydration intermediate N-( Dibenzylidene)piperidin-4-amine 103.1g, yield 78%.
[0023] Synthesis of 1-(3-methoxypropyl)piperidin-4-amine:
[0024] Under the protection of nitrogen, take the above intermediate (26.4g, 0.1mol) in a 500mL three-necked flask equipped with a temperature agent and a dropping funnel, and add 120mL of anhydrous tetrahydrofuran to completely dissolve it. 60% solid sodium hydride (6.0 g, 0.15 mol) was added in 5 batches, and after the addition was completed, the reaction was maintained at 0° C. for 30 minutes. Subsequently, 3-methoxybromopropane (...
Embodiment 2
[0026] Synthesis of N-(dibenzylidene)piperidin-4-amine:
[0027] Add 4-aminopiperidine (20.0g, 0.2mol) and benzophenone (36.4g, 0.2mol) into a 250mL reaction flask with a water separator, add 150mL of toluene and 4A molecular sieves (30g), reflux conditions Divide the water for 8-10 hours. After the reaction is completed, spin the reaction solution to dryness, add ethanol / heptane mixed solvent for recrystallization, and obtain the condensation dehydrated intermediate N-(dibenzylidene)piperidin-4-amine 36.4 g, yield 69%.
[0028] Synthesis of 1-(3-methoxypropyl)piperidin-4-amine:
[0029] Under the protection of nitrogen, take the above intermediate (26.4g, 0.1mol) in a 500mL three-neck flask equipped with a temperature agent and a dropping funnel, add 120mL of anhydrous tetrahydrofuran to dissolve completely, cool the reaction solution to 0°C, and slowly add After the addition of 2.5M n-BuLi (48mL, 0.12mol), keep the temperature at 0°C for 30 minutes. Then, 3-methoxybromopr...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com