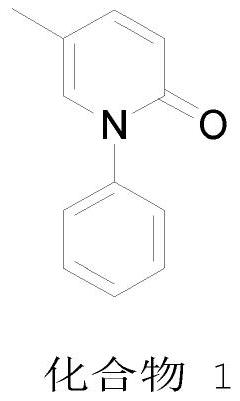
A kind of preparation method of pirfenidone
A technology for pirfenidone and a compound, which is applied in the field of preparation of pirfenidone, can solve the problems of increasing the difficulty of pollution treatment, difficulty in purchasing, multiple impurities, etc., and achieves the quality control guarantee of reaction process and final product, and the cost of starting raw materials. Inexpensive and easy to obtain, the effect of simple synthesis process
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0039] 1) Using anhydrous zinc chloride as a catalyst, diethyl malonate and 1,1,3,3-tetraethoxy-2-methylpropane were refluxed in acetic anhydride for 20h, and diethyl malonate was The molar ratio of 1,1,3,3-tetraethoxy-2-methylpropane, catalyst zinc chloride and solvent acetic anhydride is 1:1.5:0.04:3.0, after the reaction is completed, the acetic anhydride is removed by concentration under reduced pressure and low-boiling by-products, resulting in a pale yellow oily intermediate compound, diethyl 2-(3-ethoxy-2-methylallylidene)malonate.
[0040] 2) Diethyl 2-(3-ethoxy-2-methylallylidene) malonate obtained in step 1) is dissolved in solvent ethanol, 2-(3-ethoxy-2-methyl) The weight ratio of allylidene) diethyl malonate to solvent is 1:15, aniline is added to react for 5h at room temperature, then sodium ethoxide catalyst is added to reflux for 8h, 2-(3-ethoxy-2-methyl) The molar ratio of allylidene) diethyl malonate to catalyst and aniline is 1:0.1:0.8, and a cyclization rea...
Embodiment 2
[0044] 1) Using anhydrous zinc chloride as a catalyst, dimethyl malonate and 1,1,3,3-tetraethoxy-2-methylpropane are refluxed in acetic anhydride for 15h, dimethyl malonate The molar ratio of 1,1,3,3-tetraethoxy-2-methylpropane, catalyst zinc chloride and solvent acetic anhydride is 1:1.0:0.03:2.5. After the reaction is completed, the acetic anhydride is removed by concentration under reduced pressure. and low-boiling by-products, resulting in a pale yellow oily intermediate compound, dimethyl 2-(3-ethoxy-2-methylallylidene)malonate.
[0045] 2) 2-(3-ethoxy-2-methylallylidene) dimethyl malonate obtained in step 1) is dissolved in solvent ethanol, 2-(3-ethoxy-2-methyl) The weight ratio of allylidene) dimethyl malonate to solvent is 1:20, aniline is added to react at room temperature for 1h, then sodium methoxide catalyst is added to reflux for 10h, 2-(3-ethoxy-2-methyl) The molar ratio of allylidene) dimethyl malonate to catalyst and aniline is 1:0.05:1.0, and a cyclization re...
Embodiment 3
[0049] 1) Using anhydrous zinc chloride as a catalyst, dimethyl malonate and 1,1,3,3-tetraethoxy-2-methylpropane are refluxed in acetic anhydride for 8h, dimethyl malonate The molar ratio of 1,1,3,3-tetraethoxy-2-methylpropane, catalyst zinc chloride and solvent acetic anhydride is 1:0.5:0.01:2.0, after the reaction is completed, the acetic anhydride is removed by concentration under reduced pressure and low-boiling by-products, resulting in a pale yellow oily intermediate compound, dimethyl 2-(3-ethoxy-2-methylallylidene)malonate.
[0050] 2) Dimethyl 2-(3-ethoxy-2-methylallylidene) malonate obtained in step 1) was dissolved in methanol as solvent, 2-(3-ethoxy-2-methyl) The weight ratio of allylidene) dimethyl malonate to the solvent is 1:10, aniline is added at room temperature for 3h reaction, then sodium ethoxide catalyst is added for reflux reaction for 2h, 2-(3-ethoxy-2-methyl) The molar ratio of allylidene) dimethyl malonate to catalyst and aniline is 1:0.01:1.2, and a...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com