Schistosoma japonicum recombinant antigen, and preparation method and application thereof
A technology of recombinant antigen and schistosomiasis, applied in the field of bioengineering, can solve the problem of no research reports and other problems, and achieve the effect of good application value
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0039] Example 1 Expression and purification of Schistosoma japonicum recombinant antigen
[0040] 1. Method
[0041] 1.1 Construction of recombinant expression plasmid
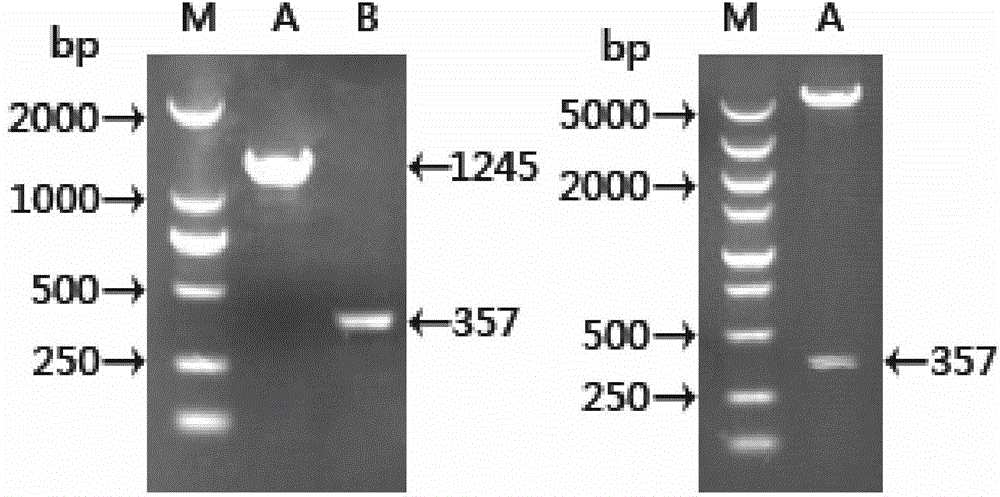
[0042] According to the gene sequence of SjTOR (AY814912.1) included in NCBI, primers were designed, upstream primer: 5'-GCGGAATTCTCGTTTCTTTTCCTA-3' (SEQ ID NO. 3); downstream primer: 5'-CGCCTCGAGGAAAGTTGTCACACAGACAGC-3' (SEQ ID NO. 4), with To amplify the SjTOR gene fragment. Using the cDNA of the 42-day worm of Schistosoma japonicum as a template, PCR amplifies the cDNA fragment containing ORF, and the reaction composition is as follows:
[0043]
[0044] PCR reaction conditions: 94°C for 5min, 94°C denaturation for 30sec, 55°C annealing for 30sec, 72°C extension for 90sec, a total of 30 cycles; finally 72°C for 15min. After the PCR product is purified, it is connected to the pMD19-T vector, transformed into DH5α cells, a single clone is picked, and the PCR bacteria liquid is identified. The positive clone is sen...
Embodiment 2
[0063] Example 2 Antigenicity detection of recombinant protein rSjTOR-ed1 of Schistosoma japonicum
[0064] 1. WesternBlotting analysis of recombinant protein antigenicity
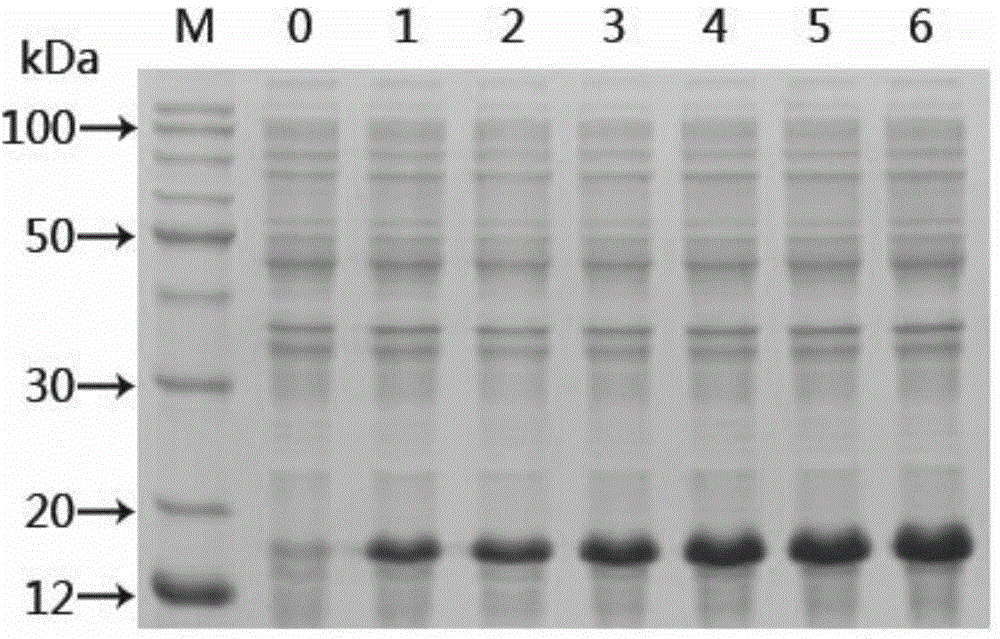
[0065] The recombinant protein rSjTOR-ed1 purified by the Ni column was subjected to SDS-PAGE electrophoresis, and then transferred to the NC membrane with 130mA for 1h at 4℃, and the antiserum against the recombinant protein was used as the first antibody to analyze the antigen of the recombinant protein Sex.
[0066] 2. WesternBlot analysis of the results of recombinant protein antigenicity
[0067] WesternBlot analysis results show that the recombinant protein rSjTOR-ed1 can be recognized by the recombinant protein immune serum and positive serum respectively, indicating that the recombinant protein rSjTOR-ed1 has good immunogenicity ( Figure 5 , 6 ). in Figure 5 Middle, M: Marker; A: Primary antibody is mouse positive serum; B: Primary antibody is normal mouse serum. in Image 6 Middle, M: Marker; A: Prima...
Embodiment 3
[0068] Example 3 Immune prevention experiment of Schistosoma japonicum recombinant protein rSjTOR-ed1
[0069] 1. Method steps
[0070] 1.1 Animal immune protection experiment
[0071] The 6-week-old BALB / c mice were divided into three groups, namely the recombinant protein SjTOR-ed1 immunization group, the 206 adjuvant control group and the PBS control group, with 10 mice in each group. Each time mice in the recombinant protein immunized group were immunized, each mouse was injected subcutaneously with 100 μL of recombinant protein SjTOR-ed1 (20 μg) and 206 adjuvant emulsion. In the 206 adjuvant control group, each mouse was injected subcutaneously with 100 μL of 206 adjuvant and PBS emulsion. In the PBS control group, each mouse was injected subcutaneously with 100 μL PBS each time. A total of three immunizations were carried out with an interval of 2 weeks each time. 7 days after each immunization, blood was collected from the orbit of each mouse, serum was collected, and store...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com