Method for separating and measuring acitretin and its genotoxic impurities
A genotoxic and acitretin technology, applied in the field of analytical chemistry, to achieve the effects of high precision and sensitivity, simple and feasible method, and high sensitivity
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
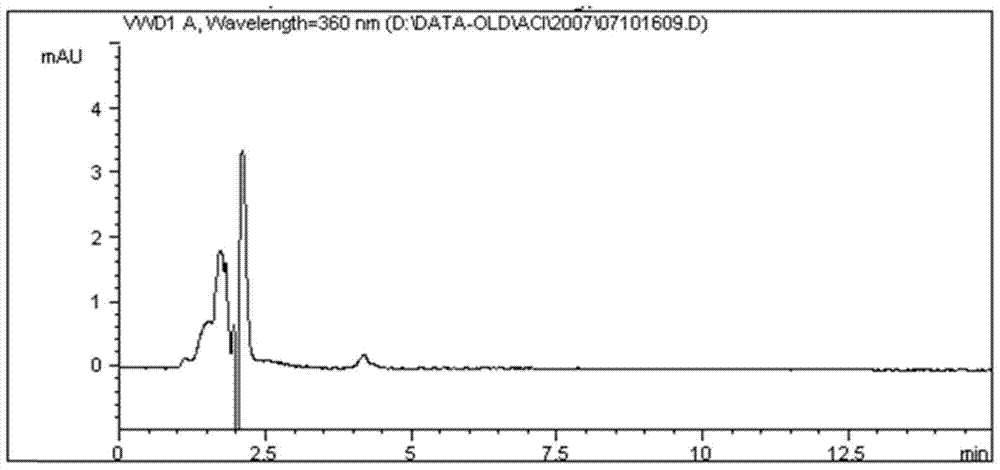
[0064] Example 1 The method for the separation and determination of genotoxic impurities in acitretin by high performance liquid chromatography
[0065] 1) Prepare a blank solution: take 10ml of tetrahydrofuran, put it in a 100ml measuring bottle, dilute to the mark with methanol, shake well, and you get it;
[0066] 2) Preparation of the test sample solution: take about 40 mg of acitretin, weigh it accurately, put it in a 100 ml measuring bottle, add 10 ml of tetrahydrofuran to dissolve it, and dilute it to the mark with methanol, shake well to obtain (0.4 mg / ml);
[0067] 3) Preparation of genotoxic impurity control stock solution: Accurately weigh 9.45 mg of acitretin methyl ester (batch number: 07032801) and 11.65 mg of acitretin butyl ester (batch number: 07032601), respectively, put them in the same 50ml measuring bottle, add 5ml of tetrahydrofuran Dissolve and dilute to the mark with methanol, shake well to obtain the control stock solution (acitretin (0.189mg / ml) and a...
Embodiment 2
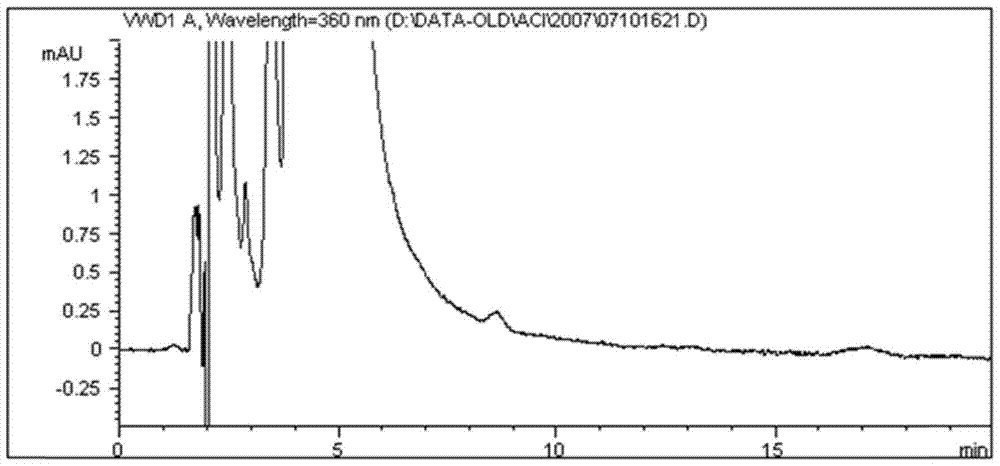
[0077] Embodiment 2 chromatographic system to the degree of separation of acitretin, acitretin methyl ester and acitretin butyl ester
[0078] 1) Prepare a blank solution: take 10ml of tetrahydrofuran, put it in a 100ml measuring bottle, dilute to the mark with methanol, shake well, and you get it;
[0079] 2) Preparation of the test sample solution: take about 40 mg of acitretin, weigh it accurately, put it in a 100 ml measuring bottle, add 10 ml of tetrahydrofuran to dissolve it, and dilute it to the mark with methanol, shake well to obtain (0.4 mg / ml);
[0080] 3) Preparation of genotoxic impurity control stock solution: Accurately weigh 9.45 mg of acitretin methyl ester (batch number: 07032801) and 11.65 mg of acitretin butyl ester (batch number: 07032601), respectively, put them in the same 50ml measuring bottle, add 5ml of tetrahydrofuran Dissolve and dilute to the mark with methanol, shake well to obtain the control stock solution (acitretin (0.189mg / ml) and acitretin b...
Embodiment 3
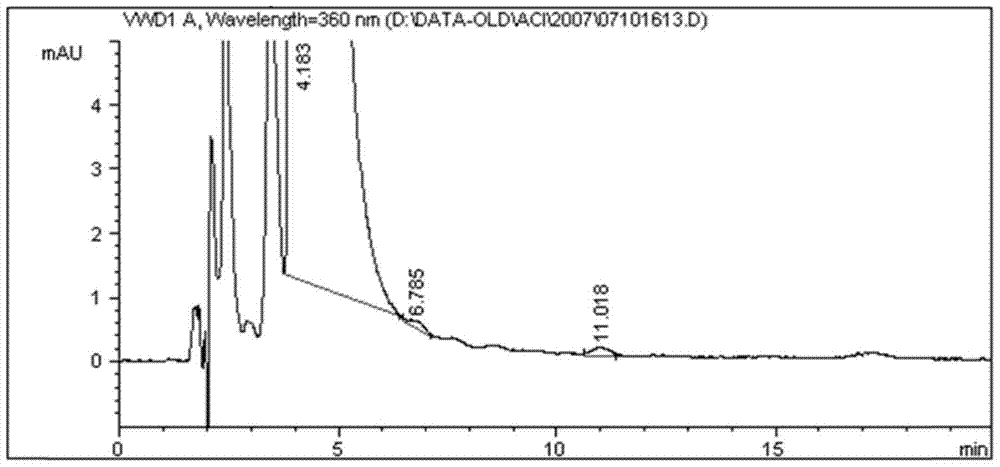
[0090] Embodiment 3 chromatographic system is to the detection level of acitretin methyl ester and acitretin butyl ester
[0091] 1) Prepare a blank solution: take 10ml of tetrahydrofuran, put it in a 100ml measuring bottle, dilute to the mark with methanol, shake well, and you get it;
[0092] 2) Preparation of the test sample solution: take about 40 mg of acitretin, weigh it accurately, put it in a 100 ml measuring bottle, add 10 ml of tetrahydrofuran to dissolve it, and dilute it to the mark with methanol, shake well to obtain (0.4 mg / ml);
[0093] 3) Preparation of genotoxic impurity control stock solution: Accurately weigh 9.45 mg of acitretin methyl ester (batch number: 07032801) and 11.65 mg of acitretin butyl ester (batch number: 07032601), respectively, put them in the same 50ml measuring bottle, add 5ml of tetrahydrofuran Dissolve and dilute to the mark with methanol, shake well to obtain the control stock solution (acitretin (0.189mg / ml) and acitretin butyl (0.233mg...
PUM
| Property | Measurement | Unit |
|---|---|---|
| wavelength | aaaaa | aaaaa |
| wavelength | aaaaa | aaaaa |
| separation | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com