A method for separating and measuring lcz696 isomer impurities
A technology for isomers and impurities, which is applied in the field of separation and determination of LCZ696 isomer impurities, can solve problems such as poor baseline, difficulty in meeting detection requirements, and poor peak shape, and achieve high column efficiency, high accuracy, and improved resolution. Effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0040] Take 50 mg of impurity AHUA, impurity AHUB, impurity AHUC, and impurity VAL-A respectively (the purity of AHUA, AHUB, AHUC, and VAL-A are all above 99%), accurately weigh, place in a 50ml measuring bottle, add methanol- Dissolve and dilute to the mark in water (volume ratio 80:20), shake well, and use it as impurity stock solution; take 50 mg of LCZ696, accurately weigh it, place it in a 50ml measuring bottle, add 0.25ml of impurity stock solution precisely, add methanol-water (Volume ratio is 80:20) dissolved and diluted to the scale, shake well, as a mixed control solution, in the solute of the mixed control solution, the concentration of LCZ696 is 1 mg / ml, and the concentration of each known impurity is 5 μg / ml.
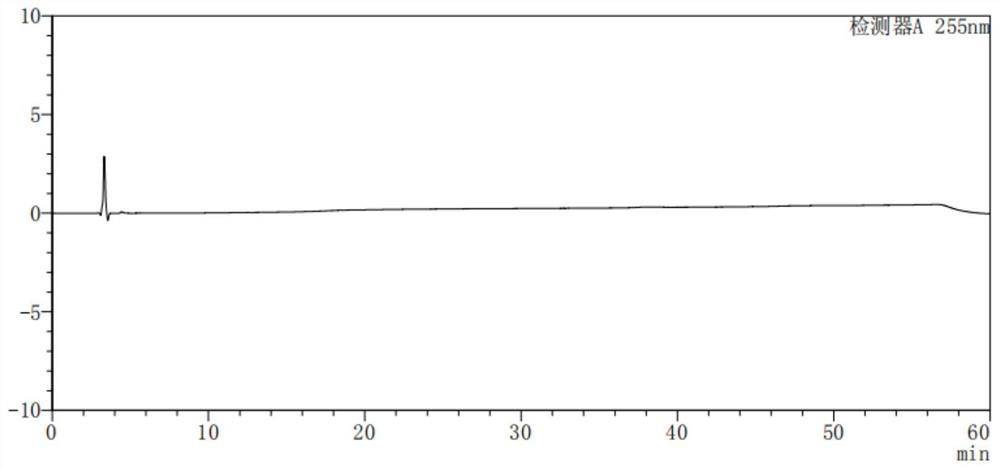
[0041] Take methanol-water (volume ratio is 80:20) and mixed control solution respectively, carry out liquid chromatography analysis according to the above-mentioned chromatographic conditions, record the chromatogram, the result is as follows: figure 1 , ...
Embodiment 2
[0044] The mensuration of embodiment two LCZ696 crude drug (provided by Chongqing Sansheng Industrial Co., Ltd.)
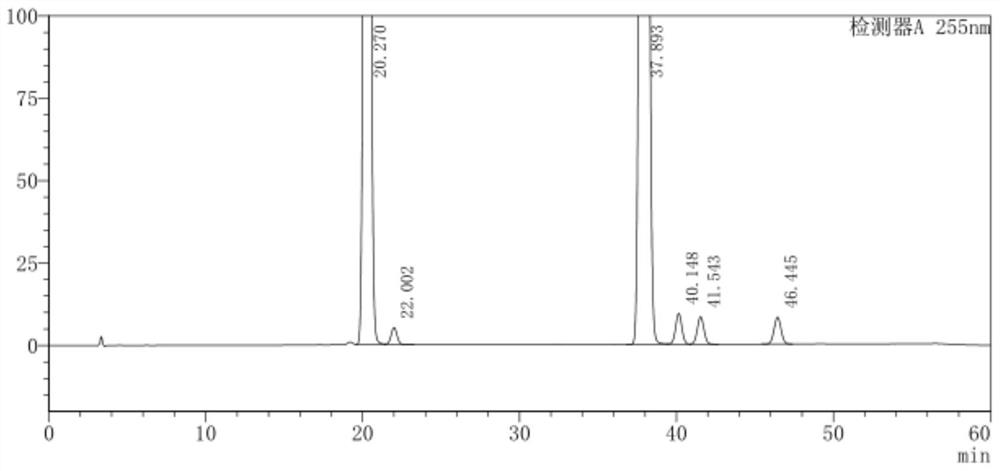
[0045] Take 50 mg of LCZ696, weigh it accurately, put it in a 50ml measuring bottle, add methanol-water (volume ratio: 80:20) for ultrasonic treatment to dissolve and dilute to the mark, shake well, and use it as the sample solution; accurately measure 1ml of the sample solution , placed in a 100ml measuring bottle, diluted to the mark with methanol-water (volume ratio of 80:20), shaken up, as a reference solution; liquid chromatography analysis was carried out according to the chromatographic conditions of Example 1. Record the chromatogram, the result is as follows image 3 , Figure 4 shown. The content of each isomer impurity in the test sample was calculated by the self-control method with a correction factor, and the test results are shown in Table 1:
[0046] Table 1
[0047] Impurities Content (wt%) VAI-A 0.0179 AHUC 0.0013 ...
Embodiment 3
[0048] The mensuration of embodiment three LCZ696 sheet (provided by Chongqing Sansheng Industrial Co., Ltd.)
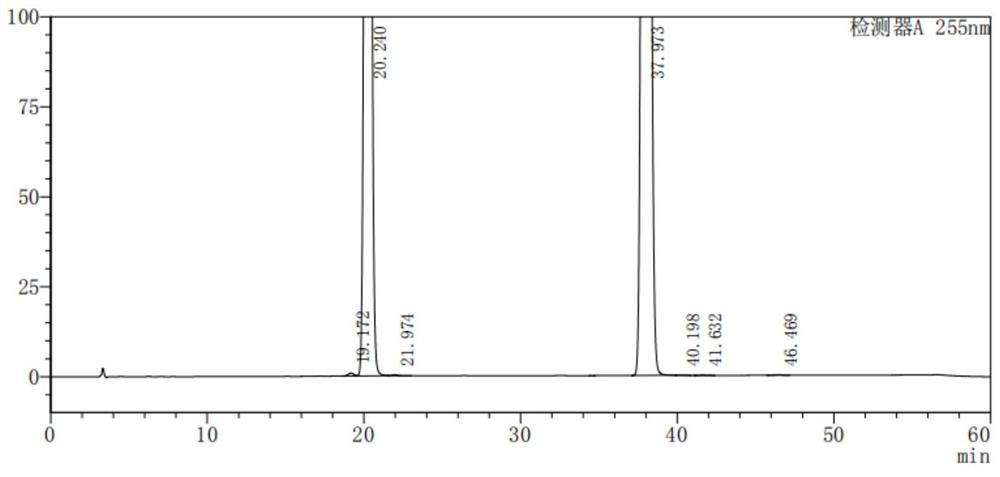
[0049] Take about 210mg of fine powder of LCZ696 tablets (approximately equivalent to 50mg of LCZ696), put it in a 50ml measuring bottle, add methanol-water (volume ratio: 80:20) sonication to dissolve and dilute to the mark, shake well, filter, and take The filtrate is used as the sample solution; accurately measure 1ml of the sample solution, place it in a 100ml measuring bottle, dilute it to the mark with methanol-water (volume ratio: 80:20), shake it well, and use it as a control solution; in addition, take it according to the prescription ratio of LCZ696 tablets An appropriate amount of blank auxiliary material is prepared, and the blank auxiliary material test solution is prepared according to the same method as the sample solution; the liquid chromatography analysis is carried out according to the chromatographic conditions of Example 1. Record the chromatogra...
PUM
| Property | Measurement | Unit |
|---|---|---|
| volume ratio | aaaaa | aaaaa |
| volume ratio | aaaaa | aaaaa |
| volume ratio | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com