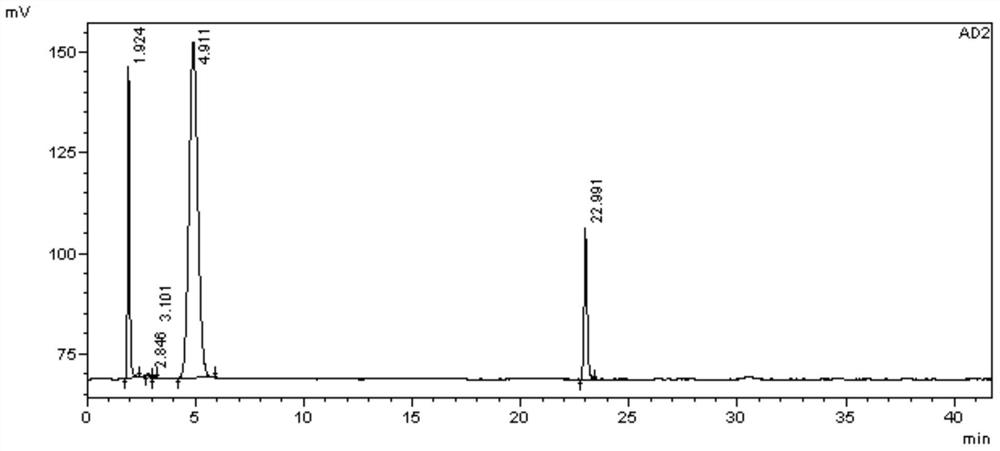
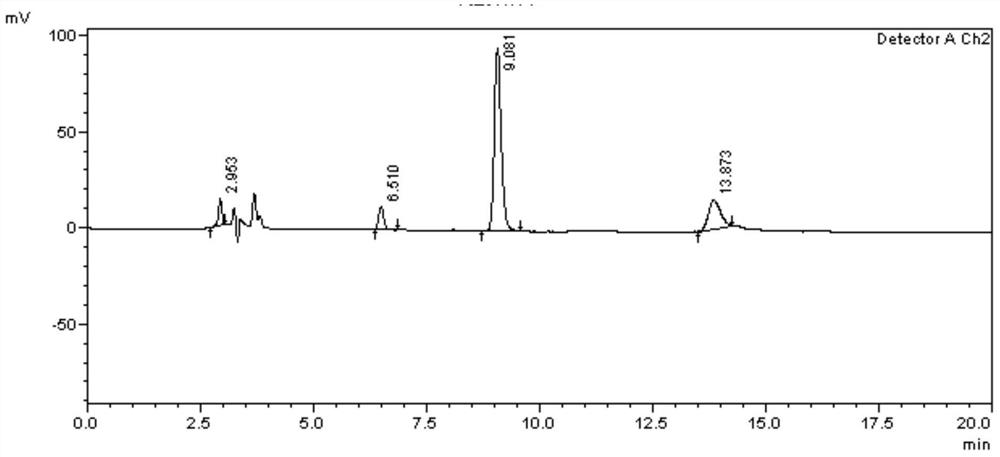
Separation and determination method of calcipotriol starting material a and related impurities
A technology of calcipotriol and impurities, applied in the field of analytical chemistry, can solve the problems of low sensitivity and inaccurate quantification of ultraviolet detectors, and achieve the effects of high sensitivity, effective separation and strong specificity
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0071] 1. Instruments and conditions
[0072] Instrument: high performance liquid chromatography;
[0073] Chromatographic column: Agilent ZORBAX SIL (4.6×250mm, 5μm);
[0074] Mobile phase: n-hexane:isopropanol=90:10(V:V);
[0075] UV detector detection wavelength: 200nm;
[0076] Column temperature: 30°C;
[0077] Flow rate: 1.0ml / min;
[0078] Injection volume: 10μl;
[0079] Diluent: The mobile phase is n-hexane:isopropanol=90:10 (V:V).
[0080] 2. Experimental steps
[0081] (1)A 1 Reference substance solution stock solution: Accurately weigh A 1 100.01mg plus diluent dissolved to make 5.05mg / mL A 1 Reference substance solution stock solution;
[0082] (2)A 2 Reference substance solution stock solution: Accurately weigh A 2 102.55mg plus diluent dissolved to make 5.12mg / mL A 2 Reference substance solution stock solution;
[0083] (3)A 3 Reference substance solution stock solution: Accurately weigh A 3 101.27mg plus diluent dissolved to make 5.06mg / mL A 3 ...
Embodiment 2
[0091] 1. Instruments and conditions
[0092] Instrument: high performance liquid chromatography;
[0093] Chromatographic column: Agilent ZORBAX SIL (4.6×250mm, 5μm);
[0094] Mobile phase: n-hexane:isopropanol=95:5(V:V);
[0095] Evaporative photodetector conditions: drift tube temperature: 51.0°C;
[0096] Gas flow rate: 1.7L / min;
[0097] Air pressure: 0.5Mpa;
[0098] Column temperature: 30°C;
[0099] Flow rate: 1.0ml / min;
[0100] Injection volume: 10μl;
[0101] Diluent: The mobile phase is n-hexane:isopropanol=95:5 (V:V).
[0102] 2. Experimental steps
[0103] (1)A 1 Reference substance solution stock solution: Accurately weigh A 1 100.01mg plus diluent dissolved to make 5.05mg / mL A 1 Reference substance solution stock solution;
[0104] (2)A 1 Positioning solution: precise measurement of A 1 Put 1.0ml of the reference substance solution stock solution in a 10ml measuring bottle, add diluent to dilute to constant volume, shake well, and obtain;
[0105] ...
Embodiment 3
[0119] 1. Instruments and conditions
[0120] Instrument: high performance liquid chromatography;
[0121] Chromatographic column: Agilent ZORBAX SIL (4.6×250mm, 5μm);
[0122] Mobile phase: n-hexane:isopropanol=99.5:0.5(V:V);
[0123] Evaporative photodetector conditions: drift tube temperature: 42°C;
[0124] Gas flow rate: 1.7L / min;
[0125] Air pressure: 0.5Mpa;
[0126] Column temperature: 30°C;
[0127] Flow rate: 1.0ml / min;
[0128] Injection volume: 10μl;
[0129] Diluent: the mobile phase is n-hexane:isopropanol=99.5:0.5 (V:V).
[0130] 2. Experimental steps
[0131] (1)A 5 Reference substance solution stock solution: Accurately weigh A 5 100.18mg plus diluent dissolved to make 0.501mg / mL A 5 Reference substance solution stock solution;
[0132] (2)A 5 Positioning solution:: Precise measurement of A 5 Put 1.0ml of the stock solution of the reference substance solution in a 10° measuring bottle, add diluent to the mark, shake well, and obtain;
[0133] (...
PUM
| Property | Measurement | Unit |
|---|---|---|
| wavelength | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com