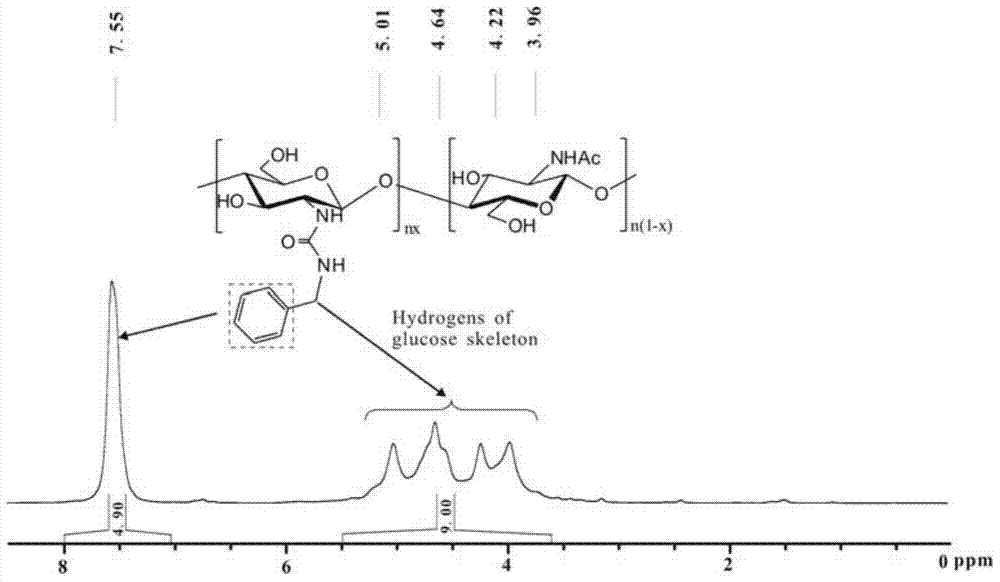
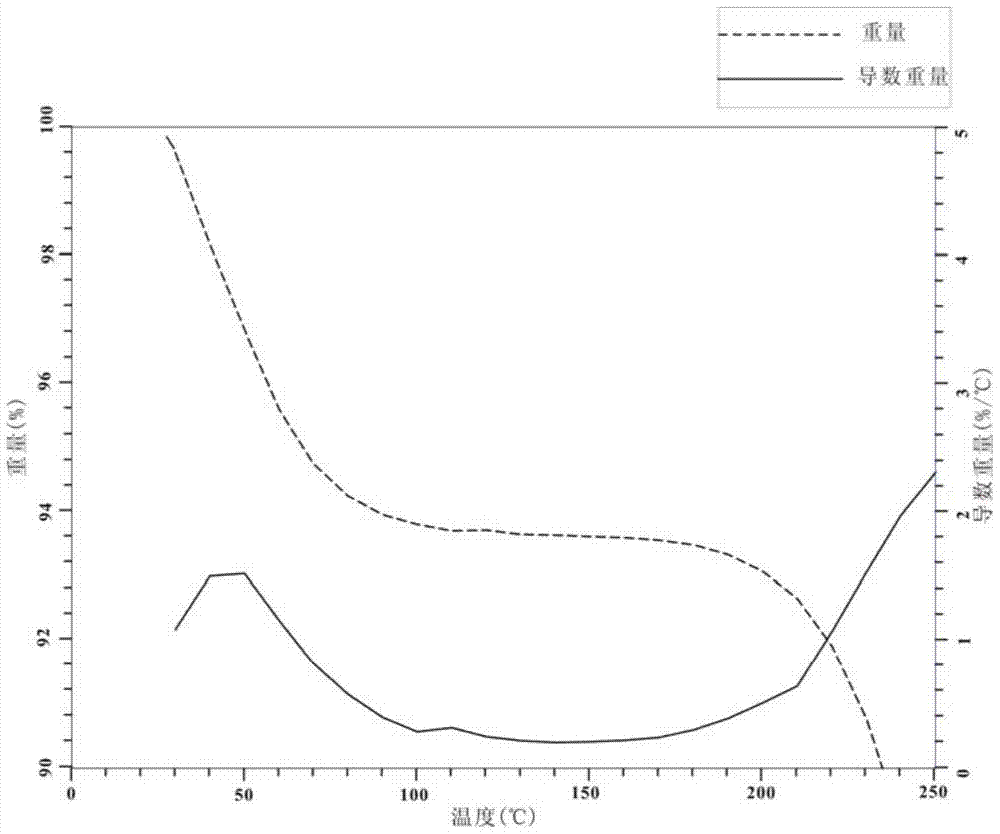
Chitosan-hydrocarbyl urea and preparation method thereof
A technology of chitosan and hydrocarbyl urea, which is applied in the field of natural polymers and their chemical modification, to achieve the effect of high degree of substitution and regular structure
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
preparation example Construction
[0031] The preparation method of chitosan used in the present invention and the measuring method of molecular weight all refer to literature [(6) Bai Zhengwu etc., chitosan-two (aryl carbamate)-(amide) and preparation method thereof [P ], application number: 201410594564.2].
[0032] The used N,N-dimethylacetamide (DMAc) of the present invention passes through before using Molecular sieves were dried three times, and LiCl was vacuum-dried at 140°C for more than 24 hours or calcined at 300°C for more than 3 hours before use.
Embodiment 1
[0034] Synthesis of Chitosan-Benzylurea:
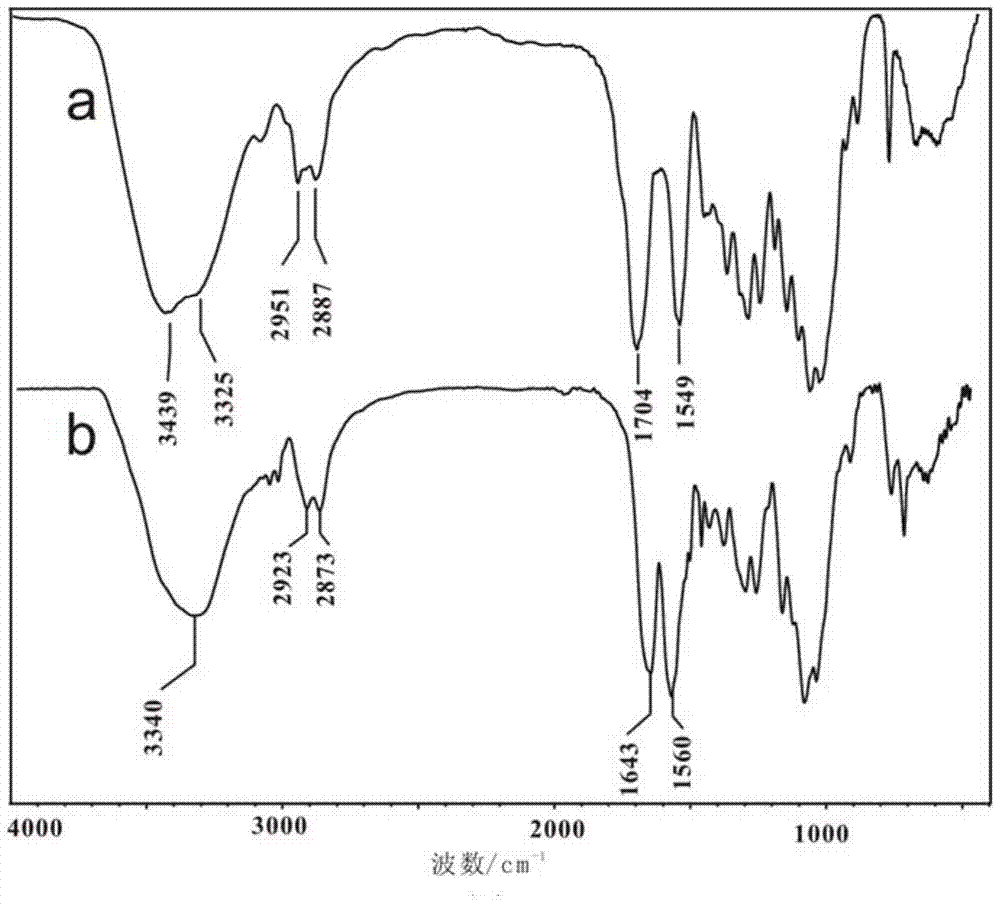
[0035]The synthesis of chitosan-N-methoxy formamide: 1.0g (6.2mmol repeating unit) chitosan (number average molecular weight 90,000, deacetylation degree is 99.2%) is joined in the 250mL there-necked bottle, add dilute hydrochloric acid Stir to dissolve chitosan until clear and transparent. Then add 35g of methanol under the ice-water bath, stir evenly, then quickly add 5.9g of methyl chloroformate (62.7mmol), control the temperature at 2-10°C for 8h, add triethylamine during the reaction to adjust the pH value of the system, and control the pH The value is between 2-7. After the reaction is complete, add 50 mL of ethanol to the reaction flask and stir vigorously, filter, wash the product with ethanol until neutral, and dry to obtain 1.27 g of chitosan-N-methoxyformamide, yield: 93%; infrared The spectrogram is attached figure 1 (a) Shown: IR(KBr,cm -1 )υ:3439,3325(-OH,-NH-),2950-2887(-C-H),1704(-COOCH 3 ), 1549 (-NH-).
[0036]...
Embodiment 2
[0038] Synthesis of chitosan-n-butylurea:
[0039] Preparation of chitosan-N-methoxy formamide: add 1.0g (6.2mmol repeating unit) chitosan (number average molecular weight 30,000, degree of deacetylation 99.7%) to a 250mL three-necked flask, add dilute hydrochloric acid and stir Dissolve chitosan until clear and transparent. Then add 28g of methanol under the ice-water bath, stir evenly, quickly add 4.82g of methyl chloroformate (51.3mmol), control the temperature at 2-8°C for 8h, add triethylamine during the reaction to adjust the pH value of the system, and control the pH The value is between 2-7. After the reaction was completed, 50 mL of ethanol was added into the reaction flask and stirred vigorously, filtered, the product was washed with ethanol until neutral, and dried to obtain 1.25 g of chitosan-N-methoxyformamide, yield: 92%.
[0040] Preparation of chitosan-n-butylurea: Take 1.2g of dry LiCl in a 50mL three-necked flask, add 15mL of dry DMAc, heat to completely di...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com